| |
Abstract
Objective: Congenital hypothyroidism is characterized by inadequate thyroid hormone production in newborn infants. Many infants with CH have co-occurring congenital malformations. This is an investigation on the frequency and types of congenital anomalies in infants with congenital hypothyroidism born from May 2006-2010 in Hamadan, west province of Iran.
Methods: The Iranian neonatal screening program for congenital hypothyroidism was initiated in May 2005. This prospective descriptive study was conducted in infants diagnosed with congenital hypothyroidism being followed up in Pediatric Endocrinology Clinicof Besat Hospital, a tertiary care centre in Hamadan. Cases included all infants with congenital hypothyroidism diagnosed through newborn screening program or detected clinically. Anomalies were identified by clinical examination, echocardiography, and X-ray of the hip during the infant’s first year of life.
Results: A total of 150 infants with biochemically confirmed primary congenital hypothyroidism (72 females and 78 males) were recruited during the period between May 2006-2010. Overall, 30 (20%) infants had associated congenital anomalies. The most common type of anomaly was Down syndrome. Seven infants (3.1%) had congenital cardiac anomalies such as: ASD (n=3), VSD (n=2), PS (n =1), PDA (n=1). Three children (2.6%) had developmental displasia of the hip (n=3).
Conclusion: The overall frequency of Down syndrome, cardiac malformation and other birth defect was high in infants with CH. This reinforces the need to examine all infants with congenital hypothyroidism for the presence of associated congenital anomalies.
Keywords: Congenital anomalies; Congenital hypothyroidism; Infants.
Introduction
Congenital hypothyroidism (CH) occurs in one out of every 2500 to 1 of 4000 neonates,1,2 but the incidence varies by geographic location and by ethnicity.2-4 The clinical importance of congenital hypothyroidism stems from it being the leading preventable cause of mental retardation. In the last decade, an unusually high frequency of extrathyroidal congenital malformations have been described in infants with congenital hypothyroidism; however, the published data vary considerably from country to country and different ethnic groups. Olivieri et al.5 reported that prevalence of additional congenital anomalies (CA) was more than four fold higher than that reported in the Italian population. The cardiac and musculoskeletal anomalies were the most frequent malformations associated with congenital hypothyroidism in the Egyptian population.6 In the study of Kreisner et al.7 13.2% of infants with congenital hypothyroidism had major congenital malformations. According to published studies, the incidence of congenital hypothyroidism is high among the Iranian population.8-10 This study was planned in order to define the frequency and type of congenital anomalies concomitant with primary congenital hypothyroidism in infants being followed up in the Pediatric Endocrinology Clinic at Besat Hospital, a tertiary care centre in Hamadan.
Methods
The Iranian neonatal screening program for congenital hypothyroidism was initiated in May 2005. This prospective cases study was conducted in Besat Hospital, Hamadan University of Medical Sciences of Iran. The study enrollment started in May 2006 and was carried out for 4 years. Cases included all CH infants diagnosed through newborn screening programs or detected clinically. The screening method was ELISA for measuring TSH on filter paper at day 3-7 of birth. Venous blood for total T4/free T4 and TSH was obtained to confirm the diagnosis for those with abnormal result (TSH>5 mIU/L). Total T4 and TSH were assessed by electrochemiluminescence. CH was defined in accordance with the guidelines of American Academy of Pediatrics (T4<6.5 μg/dL and TSH>10 mIU/mL after 1 month of age).11
All infants biochemically confirmed with CH (low T4, and TSH>10 mIU/mL in venous blood) were referred to the Pediatric Endocrinology Clinic, for starting levothyroxine therapy. Ethical approval was obtained from the local research and ethics committee at Hamedan University of Medical Sciences in 2006 and written informed consent was provided by parents. Infants were excluded if they were IUGR, premature, or a product of in vitro fertilization. Infants with panhypopitiutarism and those whose parents failed for follow-up were also excluded from the study.
At the time of enrollment, a detailed record of the required information including the neonate’s age, sex, parental consanguinity (1st cousin relation), the mother’s age at delivery, venous TSH and T4 level as well as further physical examination were made. Infants were carefully examined every two months during their first year of life. The clinical examination included looking for the presence of developmental displasia of the hip (DDH), heart murmurs, dysmorphic features, and other congenital anomalies. In the presence of heart murmur, the infants were referred to a pediatric cardiologist for echocardiography. Down syndrome diagnosis was confirmed by cytogenetic studies. DDH was detected based on clinical examination and and X-ray of hip by an orthopedic consultant. Data on CA in congenital hypothyroidism infants were obtained from a review of questionnaires based on medical records.
Chi-square test and t test or paired t test were used for non-parametric and parametric variables, respectively, using SPSS 11 software. Values are reported as Mean±SD and ranges. The data was manually extracted and displayed in a descriptive manner. Results with p-value less than 0.05 were defined as statistically significant.
Results
Overall, 157 infants with primary CH were detected among 105320 infants screened from May 2006 to 2010 in the region. This gave an incidence for CH of 1 in 658 live births. Ten infants with CH failed to complete followup protocol and were therefore excluded. Three infants who had normal tests on the newborn screen but later developed hypothyroidism were also included. The study population consisted of 150 infants with primary CH, 72 (47%) females and 78 (53%) males with mean age of 47±61 days (range: 6 days - 120 days). The mean mother’s age at delivery was 25±5.3 years (range: 17-40 years). Among the studied infants, 27.2% had parental consanguinity. The mean screening TSH and venous TSH values were 52.09±73.4 mIU/mL, median 41.5 (range: 5-333 mIU/mL) and 43.46±30.4 mIU/mL, median 40.1 (range: 10-160 mIU/mL), respectively.
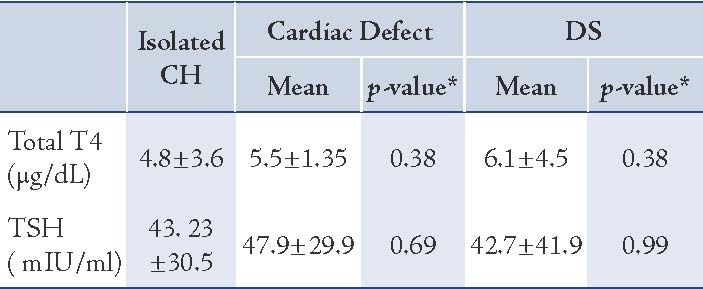
The mean value of T4 levels was 4.9±3.5 μg/dL, and median was 4.5 μg/dL (range: 0.0-14 μg/dL). In one case, the total T4 was 14 μg/dL but had low free T4. In total, 30 infants (20%) had congenital anomalies. The distribution of type and frequency of congenital anomalies of CH cases enrolled in this study are presented in Table 1. The difference in sex ratio between infants with CA and without CA was not significant (p=0.24). There was also no significant association between parental consanguinity (1st cousin parental consanguinity) and the prevalence of congenital anomalies. The mean mother’s age at delivery was not significantly associated with CA in infant with CH (p=0.11). The mean mother’s age at delivery in DS group (29 years) was not significantly higher than in infants with isolated CH (25.5 years [p=0.20]). Mean serum TSH and T4 levels in CH infants with and without CA are presented in Table 2. Mean T4 and TSH levels were not significantly different in infants with DS compared to infants without DS, (p=0.38 and p=0.99, respectively). Mean TSH and T4 levels were not significantly higher in patients with cardic defect compared to those without, (p=0.38 and p=0.69, respectively). Overall, in infants with congenital anomalies, the venous TSH levels were not significantly higher than in subjects with isolated CH (p=0.58).
Table 1: The distribution of type and frequency of congenital anomalies of infants with congenital hypothyroidism.
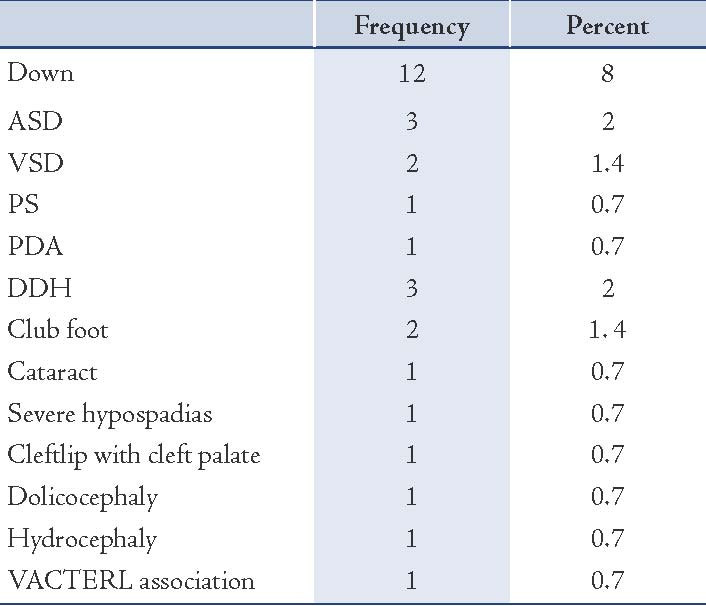
Table 2: Mean venous levels ofT4 and TSH in congenital hypothyroid (CH) infants without congenital anomalies, with cardiac defects and DS.
* T Test
Discussion
This study revealed that congenital malformations are substantially more frequent among infants with congenital hypothyroidism than expected. Down syndrome (8% vs. 0.12% in general population) being the most frequent co-occurring birth defect followed by congenital heart defects (4.9% vs. 1.7% in euthyroid controls in our country).12
The findings in this study are consistent with the high incidence of congenital anomalies other than those of thyroid gland that have been reported in numerous previous studies.13-17 For example, in a retrospective study of 1,520 patients with CH, Gu et al.13 observed that the incidences of extrathyroidal congenital anomalies (14.6%) and DS (5.7%) were significantly higher among the CH patients than among the general population. Kreisner et al.17carried out a prospective clinic-based study for evaluation of major congenital malformations in CH infants from Brazil. Among the 76 patients identified, 10 (13.2%) had major congenital malformations of whom 8 (10.5% of the total) had cardiac malformations. In a retrospective review of the CH infants, Olivieri et al.5 reported that prevalence of additional CM (8.4%) was more than four fold higher than that reported in the Italian population. Cardiac anomalies were the most frequent malformations associated with CH with a prevalence of 5.5%. Anomalies of nervous system, eyes, and multiple congenital malformations were also observed. In a study by Stoll et al.18 among 129 CH infants, 15.5% had additional anomalies of whom 6.9% had congenital cardiac anomalies. Seven of the 34 infants with congenital hypothyroidism diagnosed through Welsh screening programme had co-occurring congenital anomalies.19
The results suggest that there is high frequency of DS associated with congenital hypothyroidism and supports the hypothesis that the prevalence of congenital hypothyroidism is unusually higher in newborns with Down syndrome than in the general population.14,20-22 However, the evidence for this is unconvincing and data are controversial. Roberts et al.14 showed that infants with DS have a 35-fold increased risk for CH compared with infants in general population. Fort et al.21 observed congenital hypothyroidism to be about 28 times more common among infants with DS than in the general population with an incidence of 1%. Van Trotsenburg AS et al.23 found that in newborns with DS, the whole distribution of T4 and TSH at screening are shifted to the left and right, respectively, pointing to a mild hypothyroid state and support the existence of a DS-specific thyroid regulation disorder. On the other hand, in the study by Devos et al.24 Down syndrome was not observed in any of the cases included in the several hundred children with congenital hypothyroidism diagnosed through neonatal screening in Quebec.
As discussed above, there is a wide variation in the data regarding the thyroid function state among patients with Down syndrome, but what is clear from the review of literature available to date is that the prevalence of congenital hypothyroidism is increased in patients with Down syndrome. However, the cause of thyroid aberrations in Down syndrome infants remains unclear.21 It was also notable in this study that infants with DS had no significantly increased T4 and lower TSH values compared to those with isolated congenital hypothyroidism leading to the opinion that hypothyroidism was severe in DS. This finding is in contrast with those noted by Fort et al.21 and van Trotsenburg et al.23 regarding mild hypothyroid state in Down syndrome patients.
In the current work, the frequency of congenital heart disease was 4.9%, consistent with other previous studies of El Kholy (9.09%),6 Balestrazzi (2.1%),25 Olivieri (5.5%),5 Gu (8.9%),13 Siebner (5.8%),16 Kreisner (10.5%),17 Reddy (29%),26 and Stoll (6.9%).18 The results of this study confirm that the prevalence of cardiac defects among infants with congenital hypothyroidism is high compared to the prevalence in the general population as reported in previous studies. Developmental dysplasia of the hip present in 2% of patients is another common congenital anomaly. In this regard, the data gathered confirm and extend previous results published by Siebner et al.16 reporting that among 243 congenital hypothyroidism infants, 3.3% had congenital dislocation of the hip. This is in agreement with Fernhoff et al.27 and Sabri et al.12 but contrary to the results of Oliveri et al.5 in Italy. According to Cassio et al.28 and Grant et al.29 the hormonal deficiency was not considerably greater in patients with associated anomalies than in those with isolated CH in this study.
The underlying etiology of high frequency of congenital anomalies in congenital hypothyroidism infants remains largely unknown. However, the data presented showed that congenital hypothyroidism may be rather a part of a larger developmental syndrome resulting from some unknown insults or teratogenic effects during early embryogenesis than an isolated event. Indeed, there is an accumulating evidence indicating that gene mutations causing congenital hypothyroidism can be a cause of increased incidence of congenital anomalies in congenital hypothyroidism.30 Dentice et al.31 identified NKX2-5 as a novel gene involved in thyroidogenesis. Given that NKX2.5 is also expressed in cardiac tissues, this may explain the increased incidence of cardiac malformations in congenital hypothyroidism.
It is noteworthy that three of 160 infants had normal TSH on the newborn screen but later developed hypothyroidism. Thus, an age diagnosis ranged up to 120 days is commonly seen. It was unclear whether they were infants with delayed TSH elevation due to abnormality of pituitary-thyroid feedback regulation, transient hypothyroidism, or a mild form of permanent CH.11
This study has several limitations. First, given the fact that many of the infants included in this study may have had a transient form of congenital hypothyroidism, it is possible that the incidence of extrathyroidal congenital malformations differs in persistent congenital hypothyroidism infants. Further studies are needed to examine incidence of CA in infants with persistent CH. Furthermore, the present study describes patients who had easily recognizable birth defect in the first year of life; conditions that may appear later or are not apparent without careful observation, extensive diagnostic tests and imaging may have been missed. In addition, given the small number of infants with congenital hypothyroidism as subjects, whether the results would be sufficiently powered to be extrapolated to the large study population is unknown. Finally, it is also possible that rates for congenital heart defects have been underestimated when echocardiography was performed only for infants with cardiac murmur. It is unknown whether different results in associated congenital heart defects were observed with routine echocardiography of all studied infants.
Conclusion
This study confirmed and extended previously published reports indicating that congenital hypothyroidism is associated with various forms of co-occurring congenital anomalies. DS was the most frequently observed congenital anomaly. Following Down syndrome, congenital heart defect and DDH were the next most common defects occurring among CH infants. Clinical evaluation should be performed in all infants with congenital hypothyroidism for the presence of associated birth defects.
Acknowledgements
The authors would like to appreciate the tremendous executive help of parents of the studied infants for allowing us to complete this study. We are indebted to Dr. Tanasan for doing echocardiography. We also thank M. Shahbazi for her assistance in ascertaining and recording the patients’ data.
This work was performed with the permission of Commitee on Ethics in Medical Research School of Medicine/Hamedan University of Medical Sciences.
References
1. LaFranchi S. Congenital hypothyroidism: etiologies, diagnosis, and management. Thyroid 1999 Jul;9(7):735-740.
2. Hinton CF, Harris KB, Borgfeld L, Drummond-Borg M, Eaton R, Lorey F, et al. Trends in incidence rates of congenital hypothyroidism related to select demographic factors: data from the United States, California, Massachusetts, New York, and Texas. Pediatrics 2010 May;125(Suppl 2):S37-S47.
3. Kempers MJ, Lanting CI, van Heijst AF, van Trotsenburg AS, Wiedijk BM, de Vijlder JJ, et al. Neonatal screening for congenital hypothyroidism based on thyroxine, thyrotropin, and thyroxine-binding globulin measurement: potentials and pitfalls. J Clin Endocrinol Metab 2006 Sep;91(9):3370-3376.
4. Skordis N, Toumba M, Savva SC, Erakleous E, Topouzi M, Vogazianos M, et al. High prevalence of congenital hypothyroidism in the Greek Cypriot population: results of the neonatal screening program 1990-2000. J Pediatr Endocrinol Metab 2005 May;18(5):453-461.
5. Olivieri A, Stazi MA, Mastroiacovo P, Fazzini C, Medda E, Spagnolo A, et al; Study Group for Congenital Hypothyroidism. A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: data from the Italian Registry for Congenital Hypothyroidism (1991-1998). J Clin Endocrinol Metab 2002 Feb;87(2):557-562.
6. El Kholy M, Fahmi ME, Nassar AE, Selim S, Elsedfy HH. Prevalence of minor musculoskeletal anomalies in children with congenital hypothyroidism. Horm Res 2007;68(6):272-275.
7. Kreisner E, Neto EC, Gross JL. High prevalence of extrathyroid malformations in a cohort of Brazilian patients with permanent primary congenital hypothyroidism. Thyroid 2005 Feb;15(2):165-169.
8. Karamizaded Z, Amirhakimi GH. Incidence of congenital hypothyroidism in Fars province. Iran J Med Sci 1992;17:78-80.
9. Hashemipour M, Amini M, Iranpour R, Sadri GH, Javaheri N, Haghighi S, et al. Prevalence of congenital hypothyroidism in Isfahan, Iran: results of a survey on 20,000 neonates. Horm Res 2004;62(2):79-83.
10. Ordookhani A, Mirmiran P, Moharamzadeh M, Hedayati M, Azizi F. A high prevalence of consanguineous and severe congenital hypothyroidism in an Iranian population. J Pediatr Endocrinol Metab 2004 Sep;17(9):1201-1209.
11. Rose SR, Brown RS, Foley T, Kaplowitz PB, Kaye CI, Sundararajan S, et al; American Academy of Pediatrics; Section on Endocrinology and Committee on Genetics, American Thyroid Association; Public Health Committee, Lawson Wilkins Pediatric Endocrine Society. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006 Jun;117(6):2290-2303.
12. Sabri MR, Shahriari H, Hashemipour M. Congenital cardiac malformations in congenital hypothyroid patients in Isfahan. JRMS 2006;11(4):234-239.
13. Gu YH, Harada S, Kato T, Inomata H, Aoki K, Hirahara F. Increased incidence of extrathyroidal congenital malformations in Japanese patients with congenital hypothyroidism and their relationship with Down syndrome and other factors. Thyroid 2009 Aug;19(8):869-879.
14. Roberts HE, Moore CA, Fernhoff PM, Brown AL, Khoury MJ. Population study of congenital hypothyroidism and associated birth defects, Atlanta, 1979-1992. Am J Med Genet 1997 Jul;71(1):29-32.
15. Oakley GA, Muir T, Ray M, Girdwood RW, Kennedy R, Donaldson MD. Increased incidence of congenital malformations in children with transient thyroid-stimulating hormone elevation on neonatal screening. J Pediatr 1998 Apr;132(4):726-730.
16. Siebner R, Merlob P, Kaiserman I, Sack J. Congenital anomalies concomitant with persistent primary congenital hypothyroidism. Am J Med Genet 1992 Sep;44(1):57-60.
17. Kreisner E, Neto EC, Gross JL. High prevalence of extrathyroid malformations in a cohort of Brazilian patients with permanent primary congenital hypothyroidism. Thyroid 2005 Feb;15(2):165-169.
18. Stoll C, Dott B, Alembik Y, Koehl C. Congenital anomalies associated with congenital hypothyroidism. Ann Genet 1999;42(1):17-20.
19. Bamforth JS, Hughes I, Lazarus J, John R. Congenital anomalies associated with hypothyroidism. Arch Dis Child 1986 Jun;61(6):608-609.
20. Refetoff S. (2ds), Dumont J, Vassart G. Thyroid disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular basis of inherited disease. New York: McGraw-Hill, 2001:4029–76.
21. Fort P, Lifshitz F, Bellisario R, Davis J, Lanes R, Pugliese M, et al. Abnormalities of thyroid function in infants with Down syndrome. J Pediatr 1984 Apr;104(4):545-549.
22. Hardy O, Worley G, Lee MM, Chaing S, Mackey J, Crissman B, et al. Hypothyroidism in Down syndrome: screening guidelines and testing methodology. Am J Med Genet A 2004 Feb;124A(4):436-437.
23. van Trotsenburg AS, Vulsma T, van Santen HM, Cheung W, de Vijlder JJ. Lower neonatal screening thyroxine concentrations in down syndrome newborns. J Clin Endocrinol Metab 2003 Apr;88(4):1512-1515.
24. Devos H, Rodd C, Gagne N, et al. A search for the possible molecular mechanisms of thyroid dysgenesis: sex ratios and associated malformations. J Clin Endocrinol Metab 1. 1999;84:2502–6.
25. Balestrazzi P, Sorcini M, Grandolfo ME, Lorenzetti ME, Giovannelli G. The association between hypothyroidism and other congenital defects. The experience of the National Registry in 1987-1992. Ann Ist Super Sanita 1994;30(3):289-293.
26. Reddy PA, Rajagopal G, Harinarayan CV, et al., "High Prevalence of Associated Birth Defects in Congenital Hypothyroidism," Int J Pediatr Endocrinol. 2010;2010:940980. Epub 2010.
27. Fernhoff PM, Brown AL, Elsas LJ J. Congenital hypothyroidism: increased risk of neonatal morbility results in delayed treatment. Lancet 1987 28;1(8531):490-1.
28. Cassio A, Tato' L, Colli C, et al. Incidence of congenital malformations in congenital hypothyroidism. Screening 1994;3(4):125-130 .
29. Grant DB, Smith I, Fuggle PW, Tokar S, Chapple J. Congenital hypothyroidism detected by neonatal screening: relationship between biochemical severity and early clinical features. Arch Dis Child 1992 Jan;67(1):87-90.
30. Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature 2000 Sep;407(6801):221-226.
31. Dentice M, Cordeddu V, Rosica A, Ferrara AM, Santarpia L, Salvatore D, et al. Missense mutation in the transcription factor NKX2-5: a novel molecular event in the pathogenesis of thyroid dysgenesis. J Clin Endocrinol Metab 2006 Apr;91(4):1428-1433.
|
|