|
Introduction
Bladder cancer is one of the most common malignancies in developed countries, ranking as the sixth most frequent neoplasm.1 Although, 70% of bladder tumor patients have a superficial, noninvasive cancer, they are at high risk of recurrence, and a small number of patients will have their tumors progress and become invasive.2,3
The assessment of urinary cytology is helpful in numerous clinical conditions, particularly in the sense that the test is valuable for screening patients at high risk of urothelium malignancy and is sufficient for patient follow-up as well as control of any residual tumor. The sensitivity of urine cytology for diagnosis of tumor of the bladder was approximately 73.87% and specificity was 99.18%, with positive and negative predictive values being 84.78% and 98.36% respectively. Efficacy of this screening test was found to be 97.67%.4,5,6
Higher quality samples are obtained when specimens are collected after morning voiding and after hydration. A voided urine specimen can be collected at any convenient time of day, 3–4 hours after the patient last voided.7 Urine cytology if performed with accuracy, plays an important role in the early diagnosis of genito-urinary tumors. It offers many advantages; it is easy to perform, it is possible to repeat the examination, painless execution, non-invasive and no restricted cost.8
Urine specimens submitted to laboratories are normally contaminated with bacteria, which is a major cause of urine cell deterioration.9
The duration between collection and preparation of the sample before cellular damage occur depends on pH, protein content, enzymatic activity and the presence or absence of bacteria. It is not possible to predict these variables even in specimens from the same anatomic site.10
Regardless of the collection technique, specimens obtained from the urinary tract should be processed immediately and as quickly as possible. When the anticipated delay in processing is greater than 24 hrs, the specimen should be immediately fixed in an equal volume of 50% ethanol or Eposti’s fixative. This pre-fixation will adequately preserve the specimen for several days to minimize cellular changes complicating subsequent interpretation.11 Rapid fixation of smears is necessary to preserve cytologic details of cells. Therefore, it is important to prevent degeneration of cells by autolytic enzymes and to preserve cells as close to the living state as possible. To achieve this, a minimum of 15 minutes fixation prior to staining is essential. Prolonged fixation for several days or even a few weeks will not affect the morphology of cells.12
The purpose of this study was to determine whether structural elements of the cells can be demonstrated to be specifically concerned with certain morphological changes that are externally induced in cells due to delay in fixation. The methods for bringing into view variations in cell structure correlated with time of existing of cells before fixation. The primary outcome measure was to provide time acceptable limits for cell morphology preservation.
Methods
In this prospective study, 50 patients visiting two referral outpatient clinics for internal medicine were randomly selected and asked to take urine self-sample. Most females were complaining from vaginal discharge. Since we were interested in cell morphology, the single inclusion criterion was no history of hysterectomy. Patients with age ranging from 20 to 70 years with a mean age of 41 years were randomly selected for this study. The effect of fixation time (after collection) of urine cells on subsequent staining quality and preservation of cells was evaluated in 150 smears prepared from 50 urine samples. Three limits of fixation were applied; immediate fixation, fixation after two hours and fixation after four hours.
Each participant was asked to sign a written ethical consent form during the interview, before the specimen was taken. The informed ethical consent form was designed and approved by the ethical committee of the Faculty of Medical Laboratory Research Board, Sudan University for Science and Technology.
Fresh voided urine specimens were collected from females with vaginal discharge. Each urine sample was placed in three sterile containers. The first one was immediately centrifugated (at 1500 rpm for 10 minutes) and the deposit was smeared on to a glass slide. The second container was stored for two hours at room temperature and the third was stored for four hours, then both specimens were treated in the same way as the first one. Each smear was immediately fixed while it was wet in 95% ethyl alcohol for 15 minutes and eventually stained adopting Papanicolaou procedure. Ethyl alcohol fixed smears were hydrated in descending concentrations of 95% alcohol through 70% alcohol to distilled water, for two minutes in each stage. Then the smears were treated with Harris’ Hematoxylin for five minutes to stain the nuclei, rinsed in distilled water and differentiated in 0.5% aqueous hydrochloric acid for a few seconds, to remove the excess stain. They were then immediately rinsed in distilled water, to stop the action of discoloration. Then the smears were blued in alkaline water for a few seconds and dehydrated in ascending alcoholic concentrations from 70%, through two changes of 95% alcohol for two minutes for each change. The smears were then treated with Eosin Azure 50 for four minutes. For cytoplasmic staining, they were treated with Papanicolaou Orange G6 for two minutes, rinsed in 95% alcohol and then dehydrated in absolute alcohol. The smears were then cleared in Xylene and mounted in DPX (Distrene Polystyrene Xylene) mount. All the reagents used were from Thermo Electron Corporation, UK.
All quality control measures were adopted throughout the study procedures. Smears were initially examined by light microscope using x10 then x40. For assessment of morphological changes and the degree of the quality of the staining, each smear was assessed and given a grade (good, very good or excellent) depending on comparison of the stained smears with illustrated photomicrographs exhibiting different degrees of cell morphology preservation [available at: www.google.com.image]. Only stained smears that showed fair staining quality were assessed.
For Cytological assessment, each slide was assessed for its level of preservation and degeneration by two cytology screeners in a blinded fashion (without knowing the time of delay for each preparation). The more delay in fixation time of a smear the less well preserved it was. Six cellular features found in degenerative change were chosen for the assessment. These were; contaminant bacteria and fungi, cytoplasmic inclusions, disintegrating cytoplasm, vacuolated cytoplasm, and presence of karyolysis, karyorrhexis and pyknosis.
SPSS version 12 statistical software was used for Statistical analysis. The 95% confidence level was used. The chi square test was used to compare the differences in categorical variables between the groups. Relationships between variables were analyzed using Friedman’s non-parametric test. A p value <0.05 was considered statistically significant.
Results
Of the 50 immediately fixed urine cells, most of stained smears showed very good staining quality constituting 36 (72%) followed by good, excellent and poor constituting, 7 (14%), 3(6%) and 1(2%), respectively. All of these cells were well preserved. Furthermore, 3 (6%) specimens have showed partial degenerative changes, with deteriorated staining quality, as shown in Table1.
For the staining quality of urine cells fixed after two hours, of the 50 specimens; 14(28%) smears showed well preserved cells, 25 (50%) were partially degenerated cells and the remaining 11 (22%) contained completely degenerated cells. Out of the 14 smears with well-preserved cells; 13 (93%) were categorized as demonstrating a good staining quality and the remaining one (7%) exhibited very good staining quality. Out of the 25 smears that revealed partial degenerated cells, 18 (72%) smears showed poor staining quality and the remaining 7 (28%) exhibited good staining quality. With regards to the 11 smears with complete degenerated cells, all of them showed poor staining quality. (Table1, photomicrographs 1,2,3, and Fig. 1) .
Table 1. The staining qualities of urine cells after variable delaying periods
|
Cells Preservation
|
Excellent
|
Very good
|
Good
|
Poor
|
Total
|
|
Staining quality of immediately fixed cells
|
|
Well preserved
|
3
|
36
|
7
|
1
|
47
|
|
Partially degenerated
|
0
|
0
|
2
|
1
|
3
|
|
Total
|
3
|
36
|
9
|
2
|
50
|
|
Staining quality cells fixed after 2 hours
|
|
Well preserved
|
0
|
1
|
13
|
0
|
14
|
|
Partially degenerated
|
0
|
0
|
7
|
18
|
25
|
|
Completely degenerated
|
0
|
0
|
0
|
11
|
11
|
|
Total
|
0
|
1
|
20
|
29
|
50
|
|
Staining quality cells fixed after 4 hours
|
|
Well preserved
|
0
|
0
|
5
|
0
|
5
|
|
Partially degenerated
|
0
|
0
|
0
|
25
|
25
|
|
Completely degenerated
|
0
|
0
|
0
|
20
|
20
|
|
Total
|
0
|
0
|
5
|
45
|
50
|
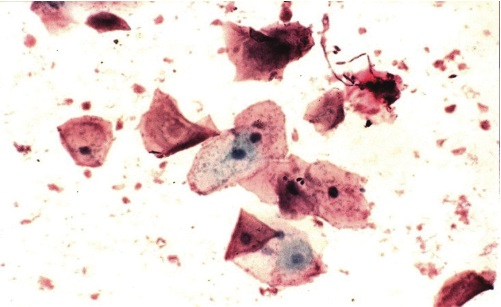
Photomicrograph 1: Well preserved cells with very good staining, Pap. Stain X4
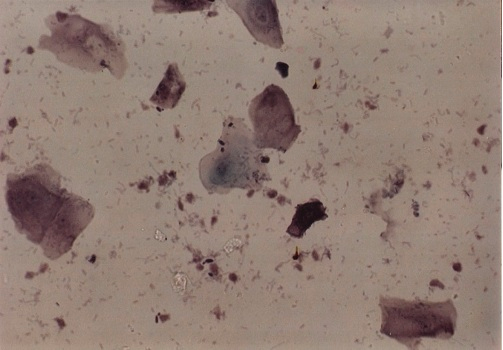
Photomicrograph 2: Partially degenerate cells with good staining, Pap. Stain X40.

Photomicrograph 3: degenerate cells with poor staining, Pap. Stain X40.
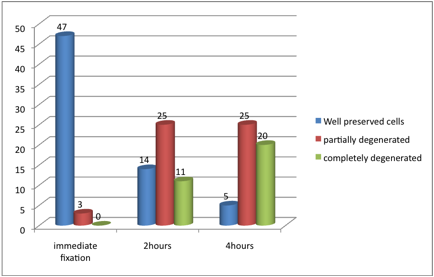
Figure 1: Cells preservation after different fixation periods
After four hours of fixation, the majority of smears contained partially degenerated cells, representing 25 (50%) followed by complete degenerated cells and well preserved cells constituting 20 (40%) and 5 (10%) correspondingly. The five well preserved smears all demonstrated good staining quality. Of the 25 specimens with partial degeneration, 24 (96%) showed poor staining quality and the remaining one was non-representative. Of the 20 smears that showed complete degenerated cells, 11(55%) showed poor staining quality and the remaining 9 (45%) were non-representative as shown in Table1.
In reference to the staining qualities in immediately fixed smears in relation to the smears fixed after 2 hours, the staining qualities for immediately fixed smears and smears fixed after 2 hours were categorized as, Excellent (3 and 0), very good (36 and 1), good (9 and 20), and poor (2 and 20) for both (immediate fixation and fixation after 2 hours) respectively as shown in Table2.
The staining qualities for immediately fixed smears and smears fixed after 4 hours were categorized as, Excellent (3 and 0), very good (36 and 0), good (9 and 5), and poor (2 and 35) for both (immediate fixation and fixation after 4 hours) respectively, as shown in Table 3.
Table 2: Shows the staining quality in immediately fixed smears in relation to the smears fixed after 2 hours.
|
Immediate fixed stain quality
|
Fixed after 2 hours stain quality
|
Total
|
|
Very good
|
Good
|
Poor
|
|
Excellent
|
1
|
2
|
0
|
3
|
|
Very good stain
|
0
|
17
|
19
|
36
|
|
Good stain
|
0
|
1
|
8
|
9
|
|
Poor stain
|
0
|
0
|
2
|
2
|
|
Total
|
1
|
20
|
29
|
50
|
Table 3: Shows the staining quality in immediately fixed smears in relation to the smears fixed after 4 hours.
|
Immediate fixed stain quality
|
Fixed after4hours stain quality
|
Total
|
|
Good stain
|
Poor stain
|
None represented
|
|
Excellent stain
|
3
|
0
|
0
|
3
|
|
Very good stain
|
2
|
29
|
5
|
36
|
|
Good stain
|
0
|
6
|
3
|
9
|
|
Poor stain
|
0
|
0
|
2
|
2
|
|
Total
|
5
|
35
|
10
|
50
|
According to the staining quality of smears fixed after 2 hours in relation to the smears fixed after 4 hours; the staining qualities for after 2 hours fixed smears and smears fixed after 4 hours were categorized as; very good (1 and 0), good (20 and 5) and poor (29 and 35) for both (after 2 hours fixation and fixation after 4 hours) respectively, as shown in Table 4. These findings indicated that the preservation of urine cells as well as their subsequent staining quality deteriorates with time and the difference was statistically significant in each 2 hours interval after the immediate time of collection, p< 0.001. (Fig. 2).
Table 4: Shows the staining quality in after 2 hours fixed smears in relation to the smears fixed after 4 hours.
|
Fixed after 2 hours stain quality
|
Fixed after 4 hours stain quality
|
Total
|
|
Good stain
|
Poor stain
|
None represented
|
|
Very good stain
|
1
|
0
|
0
|
1
|
|
Good stain
|
4
|
16
|
0
|
20
|
|
Poor stain
|
0
|
19
|
10
|
29
|
|
Total
|
5
|
35
|
10
|
50
|
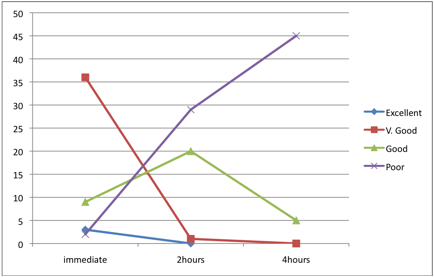
Figure 2: Staining qualities after different fixation delaying times
Discussion
There are several reasons for diagnostic inaccuracy of cytological investigations for better patient management. Voided urine cytology is used to monitor patients with suspected, primary and recurrent urothelial carcinoma.13 It is also the only non-invasive method of detecting such malignancies. Although all smears investigated in this study were found not to contain any atypical changes, the application of urine cytology to vaginal discharge is considered as the best screening device depending on the goals of the clinical practice. Therefore, it has an excellent specificity with few false-positive cases. Urine is an inhospitable environment for cells. Consequently, degenerative changes that make diagnosis difficult are common.14 Reactive changes caused by inflammation, bacterial infection, fixation and sterilization, which are responsible for most false diagnoses. The results from this study showed that there were statistically significant differences (p<0.001) between immediately fixed smears and smears fixed after 2 hours or 4 hours in preservation of the morphology of cells and staining quality. This gives good evidence about the role of early fixation and sterile containers in subsequent quality of smears. The collection of specimens under sterilization is very important to prevent disagreement between the results of the primary and subsequent examinations due to various reasons, such as the effect of bacteria propagation.9
The immediate fixation is necessary to minimize cellular changes complicating subsequent interpretation. Consequently, immediate fixation prevents the degeneration of cells by autolytic enzymes present and preservation of cells as close to the living state as possible.11
Factors such as pH, specific gravity and the duration between collection and preparation of urine have a profound effect on cell morphology and causes swelling and loss of internal morphological detail. Therefore, urine for special cytological analysis should be as fresh as possible. While a regularly voided specimen is acceptable for routine urinalysis, a midstream collection sometimes is preferred for "Obtaining a clean catch urine specimen –female."15 Corriston in 1989 studied a method for preserving cytology specimens, based on a modification of the method used for the storage of lymphocytes to preserve the cytological feature for long time. He used fetus calf serum and 20% dimethyle sulphoxide aliquot. Cytological details were well preserved in a variety of samples including fine needle aspiration, urine and bronchial brushings. The method is practical and easy to perform. However, this was not included this in the current study, as most laboratories do not include this in their analysis.
Conversely, most laboratories in Sudan are using 95% ethyl alcohol for wet preparations as preparatory for subsequent Pap. Stain, for that reason, this study applied 95% ethyl alcohol as a tested fixative. Furthermore, when the anticipated delay in preparation of urine sample is expected, the specimen should immediately be fixed in an equal volume of 50% ethanol or Eposti’s fixative.11
Conclusion
Overall, delay in urine fixation significantly deteriorates the preservation of cells as well as their subsequent staining quality. Sterility of collection container does not seem to affect the preservation and quality of staining, particularly if the preparation is instantly fixed.
Therefore, it is recommended that; urine specimens for cytology are fixed immediately without delay with the suitable fixative, the application of such study to a known pathological cell population (malignant) and normal control group is recommended to assess the influence of delay on diagnosis, and it is also recommended that a collection fluid fixative should be tested against smear fixation for urine cytology.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
|