Non-steroidal anti-inflammatory drugs (NSAIDs) comprise a group of medicines that exert their action by inhibiting cyclooxygenase (COX) isozymes. They have anti-inflammatory, analgesic, and antipyretic activities.1 They are one of the most common medications used in primary health care globally because of their low abuse potential, strong efficacy, and long-term clinical use to reduce or relieve various types of pain.1-3 They are known to reduce inflammation in osteoarthritis and musculoskeletal conditions where they work to enhance recovery and promote mobility and physical activity. NSAIDs are usually prescribed for lower back pain, osteoarthritis of the knee and shoulders, musculoskeletal trauma, rheumatology cases, dysmenorrhea, abdominal pain, headache, and high fever.4 NSAIDs, if used for the common cold, are also associated with significant benefits for outcomes related to their analgesic effects in headache, ear pain, and muscle and joint pain.5 Studies comparing the effect of NSAIDs with paracetamol in dysmenorrhea showed that NSAIDs are superior to paracetamol but have more adverse events.6 Topical NSAIDs have almost the same efficacy but only in the first two weeks of use.7-9
NSAIDs provide medical benefits, but they are well known to affect the gastrointestinal tract, kidneys, liver, and cardiovascular system adversely. Additionally, they interact with various medications, such as warfarin and aspirin.3 The National Institute for Health and Care Excellence (NICE) guideline recommended the use of paracetamol regularly with regular exercise as the first-line therapy for lower back pain and osteoarthritis. If no response occurs, NSAIDs can be used in conjunction with proton pump inhibitors in patients aged 45 years or over to reduce complications such as gastrointestinal (GI) bleeding.10,11
The use of NSAIDs in pain management is common and not usually controlled, as NSAIDs are the second-line pain management treatment after paracetamol according to the World Health Organization (WHO) pain management ladder.12
In a rheumatology clinic in the West Midlands, UK, an audit on the use of cyclooxygenase-2 (COX-2) selective NSAIDs found that 92% of patients who were taking NSAIDs should have been prescribed a COX-2-selective agent in accordance with NICE guidelines.13 In another study, NSAIDs were prescribed inappropriately for gastritis or nonspecific abdominal pain, or the indication for prescription was not clearly written.14
In Oman, there are no guidelines or protocols controlling the use of NSAIDs in primary health care institutions and their use in this setting has not been studied. As in many countries, NSAIDs are widely used by primary care physicians in Oman. Currently, the available NSAIDs in primary health care centers include ibuprofen 400mg, mefenamic acid 500mg, and diclofenac sodium 50mg, whereas both topical NSAIDs and COX-2 selective NSAIDs are not available.
We sought to assess the trend of NSAID use in four primary health care institutions in the province of A’Seeb, located in the capital city of Muscat. Additionally, we evaluated the relationship between physicians years of experience and prescription frequency as a secondary objective.
Methods
We conducted a clinical audit in four primary health care centers in the Muscat region (Al Khoudh, Al Mawaleh, Al Hail, and North Mawaleh) in the first week of April 2014. The patient population seen in these health centers represented all age groups, socioeconomic classes, and education levels. The target population for this study was patients aged 18 years and over who attended any of the four health centers and were prescribed NSAIDs by the attending general practice doctors. The prescribing doctors were not aware of the audit. Patients who were prescribed NSAIDs by a specialty clinic physician, such as a rheumatologist, were excluded. Patients were selected using systematic random sampling. Overall, 272 patients were recruited from the pharmacy and gave their informed consent. The data were collected by two methods: direct face-to-face interview of patients and evaluations of the patients’ electronic medical files. The aim of the face-to-face interview was to get any information not documented in the patient’s file and to confirm if the patient received NSAIDs from another health institution or over-the-counter. If the information obtained from the two methods were inconsistent, the information was clarified with the patient. The data were collected using a data collection sheet and included the following; patient demographics, past and current medical history of related comorbidities (diabetes mellitus, cardiovascular diseases, hypertension, gastric or duodenal ulcer, hepatic or renal impairment), NSAID type, dose, duration and indications for use, concomitant warfarin or/and aspirin prescriptions, and co-prescription of gastroprotective agents. This information addressed the risk factors for adverse GI and cardiovascular events for each patient and the possibility of a previous trial of paracetamol before prescribing NSAIDs. The collected data were analyzed using statistical software SPSS Statistics (SPSS Inc., Chicago, US) version 22. A descriptive analysis of the categorized variables was presented as proportions, and continuous variables were presented as the mean and standard deviation. The chi-square test was used to study associations.
Results
The total number of patients seen in the one-week study period in the four health centers, regardless of whether they received an NSAID prescription, was 3,524 patients. This number excludes pregnant women. Of these patients, 544 patients (15%) received an NSAID prescription. The data were collected from 272 patients who were randomly selected from the NSAID prescribed patient group; 44% were males and 56% were females. The percentage of patients who received NSAID prescriptions at each health center are shown in Table 1. Health center A had the lowest NSAID prescription proportion (9%), whereas health center C had the highest (28%). A total of 54 general physicians were involved in the audit: 18 doctors (33%) had more than 10 years of experience, 12 doctors (22%) had 5–10 years of experience, and 24 doctors (45%) had 0–5 years of experience. The highest number of NSAID prescriptions (47%) were prescribed by doctors with more than 10 years of experience, compared to the doctors who had either 5–10 years of experience or less than five years of experience [Table 2]. Most of the patients who received NSAIDs were aged 18–35 years (61%). Thirty percent were aged 36–59 years and only 9% were aged 60 years or over.
Table 1: Percentage of patients receiving NSAIDs of the total number of patients seen in four health centers in Muscat over a five-day study period.
|
A |
1186 |
102 |
9.0 |
51 |
4.0 |
|
B |
1005 |
138 |
14.0 |
69 |
7.0 |
|
C |
607 |
172 |
28.0 |
86 |
14.0 |
|
D |
726 |
132 |
18.0 |
66 |
9.0 |
HC: health center; NSAIDs: non-steroidal anti-inflammatory drugs.
Table 2: Percentage of prescriptions according to prescribing doctors’ years of experience.
|
0–5 |
45.0 |
39.0 |
|
5–10 |
22.0 |
14.0 |
Table 3: Associated risk factors in the patient sample.
|
Diabetes mellitus |
7.0 |
|
Hypertension |
14.0 |
|
GI problems |
1.0 |
|
Liver diseases |
0.4 |
|
Heart disease |
1.0 |
|
Bleeding disorders |
0.0 |
|
Renal disorders |
0.0 |
NB: some patients had more than one risk factor so the sum is more than 100%.
Seven percent and 14% of patients had diabetes and hypertension, respectively. Two patients (1%) had GI problems, and another two patients (1%) had heart disease. The remaining 84% of patients did not have any risk factors [Table 3]. The sum of percentages was more than 100% because some patients had more than one risk factor.
Table 4: Percentage of prescriptions by NSAID type.
|
Diclofenac sodium 50mg |
30.0 |
|
Mefenamic acid 500mg |
24.0 |
NSAID: non-steroidal anti-inflammatory drug.
The analysis of NSAIDs prescription indications showed that musculoskeletal problems were the main reason for prescribing NSAIDs in primary care (35%), followed by acute upper respiratory infections (AURI) and ear, nose and throat (ENT) problems (21%). Approximately 20% of all prescriptions had no indication given [Figure 1].
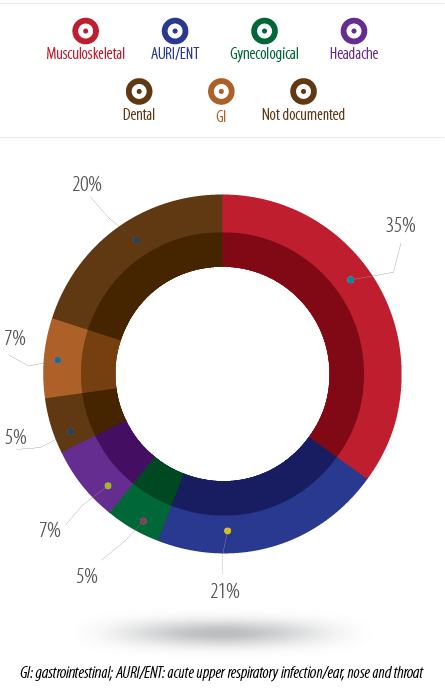
Figure 1: Percentage of NSAIDs prescriptions according to the indication.
Ibuprofen was the most frequently prescribed NSAID (46%), followed by diclofenac sodium (30%) and mefenamic acid (24%) [Table 4]. Two-thirds (67%) of patients were prescribed NSAIDs within the last 12 months. In total, 58% of patients received an NSAID prescription 1–4 times within 12 months, and 9% had received NSAIDs five or more times within 12 months. However, two patients received NSAIDs 10 and 12 times, respectively.
The patients who received NSAIDs 1–4 times within 12 months had more side effects, primarily epigastric discomfort (p=0.130). Diclofenac sodium was prescribed more often in musculoskeletal conditions (58%), whereas ibuprofen was more often prescribed in AURI/ENT conditions (52%). The mean and median durations of NSAID prescriptions of all types were 5.6 and 5.0 days, respectively, with a minimum period of one day and a maximum of 30 days.
Seven percent of patients reported side effects from previous NSAIDs use. Most had epigastric discomfort or a GI abnormality (6%). Patients prescribed ibuprofen reported more side effects (46%) than those who received diclofenac (30%) or mefenamic acid (24%); this difference was not statistically significant. The percentage of co-prescriptions of gastroprotective agents was 14%, but only 47% of the patients who had GI side effects received gastroprotective agents. Patients who received ibuprofen were more likely to receive a co-prescription of gastroprotective agents: 45% of the 14% who received gastroprotective agents compared to 31% in the diclofenac group and 24% in the mefenamic acid group (p<0.001). Further, 6% of patients who regularly took aspirin for other conditions were prescribed NSAIDs. The total number of patients who received paracetamol with an NSAID prescription was 28 (10%) and 40% of these were AURI cases with fever.
Discussion
The total number of patients seen in a one-week study period in four health centers in Muscat was 3,524. Our study demonstrated that 15% of these patients received one type of NSAID during the study period. A study in Saudi Arabia found that 24% of all prescriptions given were NSAIDs (15% were ibuprofen and 9% were diclofenac), which was slightly higher than our findings.14
The trend of prescription use differed among the institutions; health center A had the lowest number of NSAID prescriptions (9%), whereas health center C had the highest (28%). The results in health center A could be explained by an education programme on NSAIDs use that took place a few months before our study. The doctors with more than 10 years of experience prescribed more NSAIDs: no previous studies have reported this. This result was unexpected because senior doctors are expected to be more careful with NSAID prescription use due to their side effects. However, there is a need for more studies on this finding.
Some studies have shown that male patients take NSAIDs more than female patients.15,16 However, our study revealed that females were prescribed more NSAIDs than males (56% vs. 44%, respectively). This finding was similar to those of other studies.17,18 This finding may be because females need NSAIDs for gynecological reasons, such as dysmenorrhea and menorrhagia, in addition to other common conditions. Additionally, the prevalence of osteoarthritis is thought to be higher in females than in males.6,19
In this study, ibuprofen 400mg was the most common NSAID prescription (46%) followed by diclofenac sodium 50mg (30%) and mefenamic acid 500mg (24%); these results were the same as those presented in another study.19,20 However, some studies showed that diclofenac was prescribed more than other types of NSAIDs.16 A possible reason for more prescriptions of ibuprofen compared to other types of NSAIDs is its preferred use in fever, upper respiratory tract infection, and dental cases. Furthermore, among the prescriptions with undocumented indications, ibuprofen was prescribed more than diclofenac and mefenamic acid.
It is well known that NSAID use is related to cardiovascular risk factors because of its effect in increasing systolic blood pressure, particularly in high-risk groups such as diabetics, hypertensive patients, and patients with heart disease.21,22 According to previous studies, diclofenac and ibuprofen have worse cardiovascular risk profiles compared to naproxen.21-23 In this study, 15% of the patients who received an NSAID prescription had one or more risk factors, such as diabetes, hypertension, or heart disease, which could affect the patients adversely. Fortunately, none of the patients had renal problems.
The concomitant use of more than one NSAID, including aspirin, increases the risk of upper GI complications.24 In this study, 6% of the patients were taking aspirin regularly for other conditions when they were prescribed an NSAID.
In our study, 7% of the patients documented a previous history of side effects from NSAIDs. Most of them had epigastric discomfort or a GI abnormality (6%), which was similar to the results of a previous study where 24% of patients experienced indigestion/stomach pain while taking NSAIDs.25 Co-prescription of a gastroprotective agent, primarily an H2-receptor antagonist (H2RA), with an NSAID prescription occurred in only 14% of the patients. This result was similar to previous studies in which the co-prescription of gastroprotective agents was low.25,26 Additionally, most (53%) of the patients with GI problems had a history of previous side effects (gastrointestinal problems) from NSAIDs use and did not receive a gastroprotective agent. Again, this finding was similar to those of other studies.13,26 This evidence indicates the underutilization of gastroprotective measures in this high-risk group of patients. Studies have shown that proton pump inhibitors are superior to placebo in preventing NSAID-induced gastric problems.27,28 Additionally, studies showed that even if the patients stopped taking NSAIDs, a significant number of them persistently used acid-suppressive agents.29
A limitation of our study was that data was collected over the span of only one week. This meant that out data did not reflect seasonal variations of the diseases, which could further reflect NSAID use.
Future studies needed to assess the knowledge and awareness of health workers about NSAIDs uses, side effects, and cardiovascular risks to improve the use of these medications. Doctors should consider alternative medications with fewer side effects for patients who take NSAIDs. Additionally, the use of COX-2 should be considered an alternative for non-specific NSAIDs particularly in patients with GI problems. Policy development to control the prescription of these medications is crucial.
Conclusion
This study proved that the number of NSAID prescriptions across various institutes varies. This finding could reflect the level of NSAID risk awareness of the prescribing doctors in these institutes. NSAIDs were prescribed for patients with comorbidities and previously documented side effects without considering gastroprotective agents. The use of these medications should be controlled, particularly in high-risk populations.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- Brune K, Patrignani P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J Pain Res 2015;8:105-118.
- Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 2008;11(2):81s-110s.
- Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, et al; British Geriatric Society. Guidance on the management of pain in older people. Age Ageing 2013 Mar;42(Suppl 1):i1-i57.
- British National Formulary 66. BNF Section 4.7- Analgesics. Pharmaceutical Press. September 2013.
- Kim SY, Chang YJ, Cho HM, Hwang YW, Moon YS. Non-steroidal anti-inflammatory drugs for the common cold. Cochrane Database Syst Rev 2009 Jul 8;(3):CD006363.
- Marjoribanks J, Proctor M, Farquhar C, Derks RS. NSAIDs for dysmenorrhoea. Cochrane Database Syst Rev 2010 Jan 20;(1):CD001751.
- Massey T, Derry S, Moore RA, McQuay HJ. Topical Non-steroidal anti-inflammatory drugs for acute pain in adults. Cochrane Database Syst Rev 2010 Jul 16;(6):CD007402.
- Derry S, Moore RA, Rabbie R. Topical Non-steroidal anti-inflammatory drugs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2012 Sep 12;9:CD007400.
- Kelly J. Topical NSAIDs ineffective after 2 weeks. Medscape Medical News. Aug 2004. Available at http:// http://www.medscape.com/viewarticle/537883.
- National Institute of Health and Care Excellence. (2008). Osteoarthritis. CG59. London. National Institute of Health and Care Excellence.
- National Institute of Health and Care Excellence. (2009). Low back pain. CG88. London. National Institute of Health and Care Excellence.
- World Health Organization. (2009). WHO’s Pain Relief Ladder. www.who.int/cancer/palliative/painladder/en/
- Price-Forbes AN, Callaghan R, Allen ME, Rowe IF. A regional audit of the COX-2 SELECTIVE non-steroidal anti-inflammatory drugs (NSAIDs) in rheumatology clinics in the West Midlands in relation to NICE guidelines. Rheumatology (Oxford). July, 44(7)921-4 2005.
- Mohammed A. Al-Homrany, Yacoub M. Irshaid, Pharmaco-epidemiological study of prescription pattern of analgesics, antipyretics, and nonsteroidal anti-inflammatory drugs at a tertiary health care center. Saudi Med J 2007;28(3):369-374.
- Sushma M, Noel MV, Ritika MC, James J, Guido S. Cutaneous adverse drug reactions: a 9-year study from a South Indian Hospital. Pharmacoepidemiol Drug Saf 2005 Aug;14(8):567-570.
- Niyaz Alama, Alok Bhardwaj, Richa Tiwari, Shailja Sharma, Vivek Dabas. Drug utilization pattern of patients using nsaids in south delhi hospital. Int J Pharm Pharm Sci, Vol 4, Suppl 3, 703-707.
- Shi W, Wang YM, Li SL, Yan M, Li D, Chen BY, et al. Risk factors of adverse drug reaction from non-steroidal anti-inflammatory drugs in Shanghai patients with arthropathy. Acta Pharmacol Sin 2004 Mar;25(3):357-365.
- Motola D, Vaccheri A, Silvani MC, Poluzzi E, Bottoni A, De Ponti F, et al. Pattern of NSAID use in the Italian general population: a questionnaire-based survey. Eur J Clin Pharmacol 2004 Dec;60(10):731-738.
- Centers for Disease Control and Prevention 1600 Clifton Rd. Atlanta, GA 30329-4027, USA 800-CDC-INFO (800-232-4636) TTY: (888) 232-6348.
- Paul AD, Chauhan CK. Study of usage pattern of nonsteroidal anti-inflammatory drugs (NSAIDs) among different practice categories in Indian clinical setting. Eur J Clin Pharmacol 2005 Feb;60(12):889-892.
- Pope JE, Anderson JJ, Felson DT. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med 1993 Feb;153(4):477-484.
- McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med 2011 Sep;8(9):e1001098.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med 1994 Aug;121(4):289-300.
- Al-Saeed A. Gastrointestinal and Cardiovascular Risk of Nonsteroidal Anti-inflammatory Drugs. Oman Med J 2011 Nov;26(6):385-391.
- Sulaiman W, Seung OP, Ismail R. Patient’s Knowledge and Perception Towards the use of Non-steroidal Anti-Inflammatory Drugs in Rheumatology Clinic Northern Malaysia. Oman Med J 2012 Nov;27(6):505-508.
- Sturkenboom MC, Burke TA, Dieleman JP, Tangelder MJ, Lee F, Goldstein JL. Underutilization of preventive strategies in patients receiving NSAIDs. Rheumatology (Oxford) 2003 Nov;42(Suppl 3):iii23-iii31.
- Graham DY, Agrawal NM, Campbell DR, Haber MM, Collis C, Lukasik NL, et al; NSAID-Associated Gastric Ulcer Prevention Study Group. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med 2002 Jan;162(2):169-175.
- Scheiman JM, Yeomans ND, Talley NJ, Vakil N, Chan FK, Tulassay Z, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol 2006 Apr;101(4):701-710.
- Sturkenboom MC, Burke TA, Tangelder MJ, Dieleman JP, Walton S, Goldstein JL. Adher-ence to proton pump inhibitors or H2-receptor antagonists during the use of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther 2003 Dec;18(11-12):1137-1147.