Pulmonary pneumatoceles are thin-walled, gas-filled spaces within the lung that usually occur in association with bacterial pneumonia and are usually transient.1 Any number of pneumatoceles can exist at any location, except at the apices.2
In the literature, there are no reported cases of pneumatoceles formation in infants and children after cardiac surgery requiring extracorporeal membrane oxygenation (ECMO). However, there are two case reports of pneumatoceles formation following respiratory syncytial virus (RSV) pneumonitis, but they were in non-ventilated infants.3,4
The purpose of this paper is to describe the clinical course of pneumatoceles in two mechanically ventilated infants, with special emphasis on the etiology and the management.
Case Report
Case one
A 14-day-old baby weighing 3500g was admitted to the pediatric intensive care unit, Royal Hospital, Oman, after repair of total anomalous pulmonary venous connection (TAPVC). He was on ECMO for two days as it was not possible to wean him from the cardiopulmonary bypass (CPB) machine. The first chest radiograph post-surgery showed bilateral haziness but no evidence of pneumatoceles [Figure 1]. The haziness might be due to reperfusion injury secondary to CPB or pulmonary hemorrhage as a complication of ECMO. His sputum culture and bronchoalveolar lavage were negative. His conventional ventilator settings were high post decannulation from ECMO. He was on synchronized intermittent mandatory ventilation/pressure control (SIMV/PC) with the following settings: peak inspiratory pressure (PIP) 25, positive end-expiratory pressure (PEEP) 6cm H2O, respiratory rate 35 breaths/min and fraction of inspired oxygen (FiO2) 0.6. His arterial blood gas showed respiratory acidosis (pH 7.15 and pCO2 72mmHg). He was put on high-frequency oscillation ventilation (HFOV) with the following settings: MAP 20, amplitude 35, frequency 8.5 and FiO2 0.6. His blood gas had improved to pH 7.34 and pCO2 56mmHg. Seven days later, he started to develop bilateral pneumatoceles; the largest was 7.2cm×5.5cm [Figure 2], which worsened with time. Conservative management with low setup on the HFOV was given for about three weeks without improvement of the pneumatoceles size. He failed to wean from HFOV to conventional ventilation. He developed tachypnea and carbon dioxide retention with each weaning attempt.
Since he was not improving on HFOV, a size 12 gauge percutaneous intercostal chest drain (ICD) was inserted to evacuate the posteriorly located right sided pneumatocele which was demonstrated by the lateral chest radiograph. Low-pressure wall suction (10cm H2O) was applied. Following this, there was progressive shrinkage of the pneumatocele on the right side and within one day we managed to wean him from HFOV to conventional mechanical ventilation. He was successfully extubated after one week. He developed bronchopleural fistula (BPF) and the ICD continued to bubble for a total of three weeks before it was removed. He was discharged from the pediatric intensive care unit on day 56 post-cardiac surgery. A chest radiograph before discharge showed significant resolution of the pneumatoceles; however, small bilateral residual pneumatoceles persisted for few weeks before they resolved.
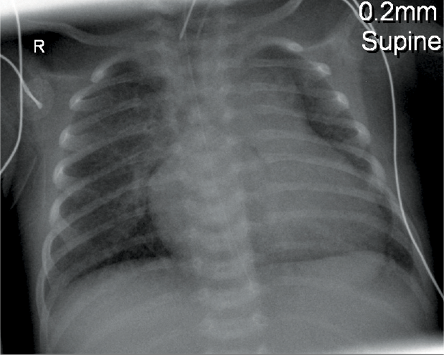
Figure 1: Chest radiograph of a 14-day-old baby (case one)immediately following cardiac surgery showed no evidence of pneumatoceles.
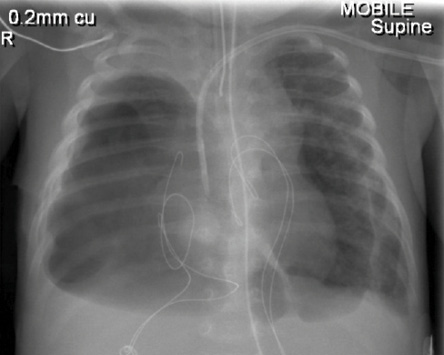
Figure 2: Chest radiograph of a 14-day-old baby (case one) showing bilateral pneumatoceles.
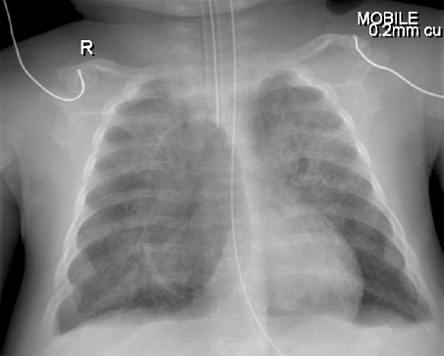
Figure 3: Right sided pneumatocele was visible by chest radiograph (case two).
Case two
A two-month-old male baby, weighing 4000g, was admitted to the pediatric intensive care unit with severe respiratory syncytial virus (RSV) pneumonitis.
His initial chest radiograph showed mild haziness bilaterally. He had severe bronchospasm which required treatment with inhaled salbutamol and ipratropium bromide along with intravenous hydrocortisone and magnesium sulfate infusion. His arterial blood gas analysis showed severe respiratory acidosis with a pH of 7.1 and a pCO2 of 90mmHg despite being on high setting (SIMV/PC/PS: PIP 26, PEEP 5, respiratory rate 35 breaths/min and FiO2 0.5). Within a few hours, he was put on HFOV, which dramatically improved his PCO2 level. Cefotaxime was administered to cover for possible bacterial pneumonia and oseltamivir phosphate for possible H1N1 infection. Sputum and blood cultures were both negative. The respiratory viral panel was positive for RSV but negative for H1N1.
Five days later, he developed right sided pneumatocele measuring 3.6cm×6.6cm [Figure 3]. He required mechanical ventilation for a total of three weeks. The pneumatocele resolved completely with conservative management on HFOV. He was found to have features suggestive of Jeune syndrome (asphyxiating thoracic dystrophy). Jeune syndrome is an autosomal recessive skeletal dysplasia characterized by a small, narrow chest and variable limb shortness with considerable neonatal mortality due to recurrent chest infections.5 Because of his severe course, immunological tests were performed and showed normal immunoglobulin levels and lymphocyte subset. The infant stayed in the pediatric intensive care unit for a total of 30 days.
Discussion
Pulmonary pneumatoceles are thin-walled, air-containing spaces that have been recognized as a possible complication of pneumonia with various infectious etiologies.6,7 The incidence of post-infectious pneumatocele formation in cases of pneumonia in children ranges between two and eight percent.8
In case one, multiple pneumatoceles were noted one week after TAPVC repair and ECMO. Unfortunately, the size of the pneumatoceles did not regress despite being on HFOV for about three weeks. There are no clear guidelines for conservative treatment versus percutaneous drainage of the pneumatoceles. However, based on our experience with this patient, percutaneous drainage should be considered when conservative management fails to show regression of the pneumatoceles. Shen et al,9 reported the successful use of HFOV in the treatment of a three-year-old child who developed large pneumatocele secondary to pneumonia. The recommended duration of HFOV use in the treatment of pneumatocele is not known. We used it for about three weeks without any improvement. Rupture of the pneumatocele will cause pneumothorax or the formation of bronchopleural fistula (BPF) especially during mechanical ventilation, which was the case with this infant.2,10,11
Ku et al,12 reported the case of a 15-month-old girl who developed multiple tension pneumatoceles secondary to pneumococcus pneumonia. She experienced severe cardio-respiratory compromise that was unresponsive to conservative treatment with HFOV. The condition was successfully treated with percutaneous intercostal drain for decompression. In another study, a 10-month-old boy had multiple tension pneumatoceles that developed four weeks after methicillin-resistant Staphylococcus aureus (MRSA) pneumonia.13 The pneumatoceles did not respond to ICD and the infant required a lobectomy.
The incidence of pneumatocele formation secondary to RSV pneumonitis is unknown. Fujii et al,14 reported the case of a one-month-old extremely premature infant with respiratory distress syndrome and enlarging pneumatocele secondary to respiratory syncytial viral pneumonitis, which was successfully managed with the placement of a percutaneous pigtail catheter.
In our second case, the pneumatocele resolved without the need for ICD. The child was treated conservatively with HFOV. It is a well-known fact that HFOV decreases airway pressures, reduces the risk of barotrauma, and improves ventilation/perfusion matching and gas exchange.15 Hence, it is possible that the use of HFOV in this patient contributed to the resolution of the pneumatocele. Therefore, it is wise to try HFOV in mechanically ventilated patient with pneumatocele. However, if the use of HFOV fails to resolve the pneumatocele or if the patient showed hemodynamic instability, then an ICD must be inserted.
It is difficult to say how long we should continue with conservative treament. In the first case, we continued for three weeks before the use of an ICD. He had already failed to wean from HFOV four times.
In the second case, the pneumatocele resolved spontaneously within one week of conservative management with HFOV. Therefore, it might be logical to give the pneumatocele some time, which might be weeks, to resolve by the use of conservative measures before the use of ICD, as long as the patient’s condition allows.
Conclusion
Pneumatocele formation is a serious complication following bacterial or viral infections. Conservative management is preferred to prevent the formation of bronchopleural fistula secondary to ICD and, thus, the need for prolonged hospital admission. However, the role of conservative management in mechanically ventilated patients is still not clear. We suggest a trial of conservative management with HFOV in mechanically ventilated stable patients for two to three weeks. However, if this fails then evacuation of the pneumatoceles is mandatory to allow weaning from mechanical ventilation and avoid hemodynamic deterioration.
Disclosure
The authors declared no conflict of interests.
Acknowledgements
The authors thank all the health care professionals who were involved in the care of the two patients.
references
- Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. AJR Am J Roentgenol 1984 Sep;143(3):509-517.
- Galea MH, Williams N, Mayell MJ. Traumatic pneumatocele. J Pediatr Surg 1992 Dec;27(12):1523-1524.
- Nwokoro C, Issa M, Nastar A, Leigh M, Ross Russell R. Pneumatoceles following bronchiolitis in infancy: Worth a second look? ERS congress. September 20th, 2010.
- Plesca D, Hurduc V, Davitoiu A, Cavache A. Rare complication of acute bronchiolitis in infants: pneumatoceles. 15th ICID. Thailand; June 13-16, 2012.
- O’Connor MB, Gallagher DP, Mulloy E. Jeune syndrome. Postgrad Med J 2008 Oct;84(996):559.
- Dines DE. Diagnostic significance of pneumatocele of the lung. JAMA 1968 Jun;204(13):1169-1172.
- Sewall LE, Franco AI, Wojtowycz MM, McDermott JC. Pneumatoceles causing respiratory compromise. Treatment by percutaneous decompression. Chest 1993 Apr;103(4):1266-1267.
- Amitai I, Mogle P, Godfrey S, Aviad I. Pneumatocele in infants and children. Report of 12 cases. Clin Pediatr (Phila) 1983 Jun;22(6):420-422.
- Shen HN, Lu FL, Wu HD, Yu CJ, Yang PC. Management of tension pneumatocele with high-frequency oscillatory ventilation. Chest 2002 Jan;121(1):284-286.
- Zuhdi MK, Spear RM, Worthen HM, Peterson BM. Percutaneous catheter drainage of tension pneumatocele, secondarily infected pneumatocele, and lung abscess in children. Crit Care Med 1996 Feb;24(2):330-333.
- McGarry T, Giosa R, Rohman M, Huang CT. Pneumatocele formation in adult pneumonia. Chest 1987 Oct;92(4):717-720.
- Ku SW, Yu TC, Chan KW. Decompression of multiple tension pneumatoceles in a child using computed tomography-guided percutaneous catheter placement. Can Respir J 2011 Nov-Dec;18(6):e82-e85.
- Wu ET, Chen JS. Management of multiple tension pneumatoceles refractory to tube thoracostomy decompression. Ann Thorac Surg 2006 Apr;81(4):1482-1484.
- Fujii AM, Moulton S. Percutaneous catheter evacuation of a pneumatocele in an extremely premature infant with respiratory failure. J Perinatol 2003 Sep;23(6):516-518.
- Baumann MH, Sahn SA. Medical management and therapy of bronchopleural fistulas in the mechanically ventilated patient. Chest 1990 Mar;97(3):721-728.