Primary immune thrombocytopenia (ITP) is a common autoimmune disease characterized by isolated low platelet count and an increased risk of bleeding in the absence of any other obvious underlying cause of thrombocytopenia.1,2 In adults, ITP typically follows a chronic course.2,3 Most patients present with mucosal bleeding or excessive bruising, and seek medical attention. However, the risk of developing serious intracranial bleeding is high when platelet count reaches less than 10x109/L.4,5 Most adults with ITP will achieve an initial response to a corticosteroid-based treatment. Unfortunately, many patients will either relapse after tapering steroid dose or develop significant side effects following prolonged use of relatively higher doses of steroids.6 Therefore, a second-line therapy is frequently needed. There are several second-line therapies: spelenectomy, rituximab, danazol, azathioprine, and thrombopoietin agonists such as eltrombopag and romiplostim.2,7-9
Rituximab is a chimeric monoclonal antibody targeting a B-cell antigen (CD20) that has been used widely as a second line therapy for ITP with a promising response rate approaching 60% in some studies.2,10-13 A response is usually seen between week one to eight of therapy and could last for five years in some patients.2,10 Although the optimal dose of rituximab is still unknown, most studies used 375mg/m2 weekly for four doses. Its safety and tolerability profile has led to its preference by many physicians and patients as a second-line therapy over splenectomy.14
In this study, we planned to estimate the response rate and the duration of response of rituximab in patients with chronic ITP treated in the two main tertiary centers in Oman and to evaluate the time to loss of response for those who achieved an initial response.
Methods
We performed a retrospective analysis of consecutive patients with ITP treated with at least one course of rituximab as a second-line therapy from the two main tertiary care hospitals in Oman (Royal Hospital and Sultan Qaboos University Hospital) between January 1995 and June 2011. The data was collected from the hospital information systems after ethical approval of the study was obtained. Collected data included age at diagnosis, gender, comorbid disorders, date of diagnosis, presenting features, initial treatments, blood counts, autoimmune workup, date of rituximab first dose, response to treatment, duration of response, and any subsequent treatments for patients who failed rituximab therapy. Rituximab was given in relapse or after discontinuation of immunosuppressive drugs due to intolerable side effects. Patients received intravenous rituximab at a dose of 375mg/m2 repeated once weekly for four weeks. The patients were monitored closely for the first 15 minutes for any signs of transfusion reactions. Premedication with oral acetaminophen and diphenhydramine were given to all patients.
We used the American Society of Hematology Response Criteria to define the responses.1 Complete response (CR) was defined as achievement of a platelet count of more than 100x109/L with no bleeding. Partial response (PR) was defined as achievement of a platelet count of more than 30x109/L with at least a two-fold increase from baseline, and absence of bleeding. Time to response was calculated from starting rituximab to the achievement of CR or PR. No response was defined as a platelet count below 30x109/L or less than a two-fold increase from baseline platelet count, or the presence of bleeding. Loss of CR or PR was defined as failure to satisfy the above definitions after the achievement of CR or PR, respectively.
Continuous variables were summarized as means with ranges (or medians when not normally distributed) and categorical variables were summarized as proportions with 95% confidence interval (CI) for the outcome variables. The Kaplan-Meier method was used to estimate the cumulative response rate and median time to respond and to lose response. Log-rank test was used to compare groups and Cox regression to assess predictors and adjust for baseline differences. All tests were two sided and we used alpha error threshold of 0.050. All descriptive, graphical and analytical statistics were done using STATA 11 software (StataCorp. 2009) for all statistical analyses.
Results
Thirty-two consecutive patients (females=20, males=12) with chronic ITP (including six patients with secondary ITP, four patients with systemic lupus erythematosus, one patient with Hepatitis C, and one patient with glomerulonephritis) who received at least one course of rituximab were included. The median age at diagnosis was 25 years (range 14–58). Patients received a median of one line of therapy before rituximab (range 1–4). Three patients failed splenectomy before starting rituximab. The median time from diagnosis to receiving rituximab was 21 months (range 1–177). No patient had human immunodeficiency virus or Hepatitis B and only one had Hepatitis C. Anti-nuclear antibody was positive in 34% of patients. The median hemoglobin level and platelet count at diagnosis were 12g/dL (range 5–16) and 11x109/L (range 1–60), respectively. The median follow-up time after starting rituximab was 26 months.
In this study, 19 of the total 32 patients had an initial response (59%) and the remaining 13 patients did not respond to rituximab. However, six of those 19 patients lost their response, leaving 13 patients with long-lasting remissions. The overall cumulative response rate was 59% (15% PR, 44% CR). The median time to respond was 30 days [Figures 1 and 2] with a response rate of 44% at four weeks (95% CI 29–62%). In patients who had an initial response, the cumulative rate of loss of response was 32% (6 of 19) with a median time to lose response of 54 months for all initial responders [Figure 2].
For those who lost their response after an initial response, the median time to lose response was 17 months. Of those who lost response, four received a second course of rituximab and all four achieved a complete response, one underwent splenectomy and one has gone off treatment with platelet counts maintained above 20x109/L. There were no significant side effects reported in this study.

Figure 1: The cumulative response rate after rituximab treatment.
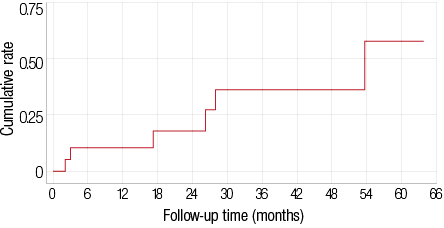
Figure 2: The cumulative rate of loss of response after an initial response.
Discussion
In this study, we confirmed the high response rate to rituximab in patients with ITP used as a second-line treatment, as reported by other studies.11,15,16 We showed that the responses can be maintained for a long duration and a second response to repeated therapy is usually achieved. Several previous studies have reported high initial response rates to rituximab. A recently published study, reported an initial overall response rate of 57% in adults and children treated with rituximab in chronic ITP.15 This response rate was confirmed by a recent systematic review and by a meta-analysis.11,16 Schweizer C et al,17 reported an overall response rate of 64% in another smaller study of 14 patients. However, in this small study there was a low rate of long remissions after an initial response. In our study, we reported a similar high overall response of 59%.
Responses in our patients lasted relatively longer than other studies. As mentioned above, in the study by Schweizer and colleagues,17 the maximum response duration was 36 months. In the systematic review by Donald M. Arnold, 11 the response lasted from two to 48 months. In our study, the median remission duration after an initial response was 54 months. The longer remission duration seen in our study is likely to be related to the younger patient sample and the early use of rituximab in the course of their treatment.
Six patients lost their response after initial successful therapy with rituximab; four were retreated and all responded. One patient went for splenectomy and the last patient maintained platelet counts above 20x109/L without any treatment. The high response rate to a second course of rituximab is reported in the literature, approaching 75% in some studies.18 The literature review by Perrotta, 19 showed that the duration of the second response was at least as durable as the first response.
The small sample size and the heterogeneous study population are two major limitations of our study. In addition, we cannot rule out selection bias given the retrospective design of the study. However, the similarities between our results and other larger and some prospective trials confirm our results and support our conclusions. The need for larger studies comparing rituximab to other second-line therapies (e.g. splenectomy or the new thrombopoietin analogues) cannot be overemphasized.
Conclusion
The response to rituximab in the second-line management of chronic ITP is high and could be long-lasting. This study was limited by its small sample size and it is difficult to generalize these results without further larger prospective randomized studies.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood [Internet]. 2009 Mar 12;113(11):2386–2393. Available at http://www.ncbi.nlm.nih.gov/pubmed/19005182. Accessed May 27, 2014.
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010 Jan;115(2):168-186.
- Stasi R, Evangelista ML, Stipa E, Buccisano F, Venditti A, Amadori S. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost 2008 Jan;99(1):4-13.
- Butros LJ, Bussel JB. Intracranial hemorrhage in immune thrombocytopenic purpura: a retrospective analysis. J Pediatr Hematol Oncol 2003 Aug;25(8):660-664.
- Iyori H, Bessho F, Ookawa H, Konishi S, Shirahata A, Miyazaki S, et al; Japanese Study Group on childhood ITP. Intracranial hemorrhage in children with immune thrombocytopenic purpura. Japanese Study Group on childhood ITP. Ann Hematol 2000 Dec;79(12):691-695.
- McCrae K. Immune thrombocytopenia: no longer “idiopathic”. Cleve Clin J Med [Internet]. 2011 Jun;78(6):358–373. Available at http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3410635&tool=pmcentrez&rendertype=abstract. Accessed May 27, 2014.
- Fogarty PF, Segal JB. The epidemiology of immune thrombocytopenic purpura. Curr Opin Hematol 2007 Sep;14(5):515-519.
- Corman SL, Mohammad RA. Eltrombopag: a novel oral thrombopoietin receptor agonist. Ann Pharmacother 2010 Jun;44(6):1072-1079.
- Imbach P, Crowther M. Thrombopoietin-receptor agonists for primary immune thrombocytopenia. N Engl J Med 2011 Aug;365(8):734-741.
- Stasi R, Pagano A, Stipa E, Amadori S. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adults with chronic idiopathic thrombocytopenic purpura. Blood 2001 Aug;98(4):952-957.
- Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med 2007 Jan;146(1):25-33.
- Wang J, Wiley JM, Luddy R, Greenberg J, Feuerstein MA, Bussel JB. Chronic immune thrombocytopenic purpura in children: assessment of rituximab treatment. J Pediatr 2005 p Feb;146(2):217-221.
- Braendstrup P, Bjerrum OW, Nielsen OJ. Jensen B a, Clausen NT, Hansen PB, et al. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adult refractory idiopathic thrombocytopenic purpura. Am J Hematol [Internet]. 2005 Apr;78(4):275–280. Available at http://www.ncbi.nlm.nih.gov/pubmed/15795920. Accessed May 27, 2014.
- Warkentin TE, Arnold DM. “Spare-spleen-uximab” for chronic ITP. Blood [Internet]. 2008 Aug 15;112(4):925–926. Available at http://www.ncbi.nlm.nih.gov/pubmed/18684872. Accessed 2014 May 27, 2014.
- Patel VL, Mahévas M, Lee SY, Stasi R, Cunningham-Rundles S, Godeau B, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood 2012 Jun;119(25):5989-5995.
- Auger S, Duny Y, Rossi JF, Quittet P. Rituximab before splenectomy in adults with primary idiopathic thrombocytopenic purpura: a meta-analysis. Br J Haematol 2012 Aug;158(3):386-398.
- Schweizer C, Reu FJ, Ho AD, Hensel M. Low rate of long-lasting remissions after successful treatment of immune thrombocytopenic purpura with rituximab. Ann Hematol 2007 Oct;86(10):711-717.
- Hasan A, Michel M, Patel V, Stasi R, Cunningham-Rundles S, Leonard JP, et al. Repeated courses of rituximab in chronic ITP: Three different regimens. Am J Hematol [Internet]. 2009 Oct;84(10):661–665. Available at http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2783818&tool=pmcentrez&rendertype=abstract. Accessed May 27, 2014.
- Perrotta AL. Re-treatment of chronic idiopathic thrombocytopenic purpura with rituximab: literature review. Clin Appl Thromb Hemost 2006 Jan;12(1):97-100.