Acute myeloid leukemia (AML) is a diverse type of blood cancer characterized by the arrest of immature clonal myeloid cells’ development and proliferation. AML is more common, and fatalities are more common as people age.1 The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway is essential for cell proliferation, differentiation, and survival in AML.2 The expression of JAK/STAT signalling proteins in AML patients has attracted increasing attention recently. The pathway is one of the signal transduction pathways that are hyperactive in about two-thirds of AML patients. According to reports, AML blasts have constitutive phosphorylation of JAK2, STAT3, and/or STAT5, which is linked to a shortened time to relapse and a bad result.3,4 Increased levels of phosphorylated JAK2, STAT3, and STAT5 are seen in vitro in 44–100% of bone marrow samples taken from AML patients.5 JAK/STAT signalling protein expression has been found to be different in AML patients. In a variety of solid tumors and hematological malignancies, including AML, STATs, especially STAT3 and STAT5, are hyperactivated, promoting cellular proliferation and survival.6 In 44–76% of AML patients, STAT3 and STAT5 are constitutively activated,7 and those with constitutive STAT3 activity have shorter disease-free survival than those without.8 We hypothesized that a higher expression of JAK/STAT proteins predicts a higher impact on overall survival (OS). The aim of this study was to evaluate JAK/STAT pathway protein expression levels in AML patients and the impact on clinical outcomes. Specifically, we planned to assess the difference of overexpression percentage of pSTAT3, pSTAT5/STAT5, pJAK2, pJAK1, and JAK1 in peripheral blood or bone marrow between diagnosis and remission, and to correlate the expression of all these proteins with OS and complete remission.
Methods
Adult patients who were diagnosed with AML based on the World Health Organization 2016 criteria were included in this study. Patients received standard intensive chemotherapy if they were fit, or azacytidine. All patients gave their informed consent before sample collection. Peripheral blood and bone marrow samples were collected at diagnosis, remission, and relapse (if this occurred during the follow-up period). Normal peripheral blood samples were obtained from healthy donors. The study was conducted in accordance with the approved protocol from the ethical committee at Sultan Qaboos University.
The collected samples were immediately processed. Mononuclear cells were isolated using Ficoll-Hypaque separation following the instructions of the manufacturer.9 Total protein was extracted from the bone marrow and peripheral blood samples using a lysis buffer containing protease and phosphatase inhibitors. The protein concentration in the samples was assessed using Nanodrops (Spectrophotometer ND-1000; version 3.3.0, United States). The cell lysates were stored at -80 oC for later use.
Protein expression was determined using enzyme-linked immunosorbent assay (ELISA) kits (Invitrogen, USA), in accordance with the recommendations of the manufacturer. The relevant ELISA kit was used to measure the following parameters, the source and catalogue number are provided in parentheses, pJAK2 [pY1007/pY1008], STAT3 [pY705], STAT5 alpha (Total/Phospho) (Invitrogen, Carlsbad, CA, United States; Cat #: KHO5621, KHO0481, Cat #: 85-86113-11), pJAK1 (Y1022), and total JAK1 (Abcam; Cat #: ab279838). In brief, the standard was diluted before adding it to the wells along with the samples and a blank. The plate was then incubated for one hour at 37 °C. After incubation, the liquid was discarded and the plate was rinsed four times with buffer. The plate was then pat-dried. The chromogenic reagent was added to the wells and the plate was incubated for 15 minutes at 37 °C in the dark. After incubation, the stop solution was added and the absorbance at 450 nm was measured within 10 minutes (Biomak, Beckman, United States).
For every set of identical standards and samples, the average absorbance values were calculated. The mean absorbance for each standard concentration was then plotted against the concentration of JAK/STAT to produce a standard curve. A best-fit curve was created through the graph’s points to guarantee correct portrayal. The mean absorbance value on the ordinate was first determined to ascertain the concentration of JAK/STAT for each sample. On the way to the standard curve, a horizontal line was drawn from this location. After marking the junction, a vertical line was drawn from the intersection to the abscissa (x-axis); this location was used to read the matching JAK/STAT concentration. To account for any performed dilutions, the concentration determined from the standard curve was multiplied by the dilution factor. Additionally, the concentration of each protein was normalized to the total protein.
Data were expressed as medians with IQR for continuous variables and as proportions for categorical variables. The overexpression of proteins was defined as 1.5 or more-fold increase in the expression of protein compared with control. The OS probability was estimated using Kaplan-Meier curve. The level of expression was compared using Wilcoxon signed-rank test for continuous variables and Chi-square test for categorical variables. The Cox regression model was used to compare survival between groups. An alpha threshold of 0.05 was used for statistical significance.
The R Foundation for Statistical Computing Platform, version 3.5.1 and GraphPad Prism® software package, version 6 (GraphPad Software Inc., CA, USA) was used to produce all descriptive and analytical results.
Results
We analyzed the expression levels of JAK/STAT pathway proteins in 23 AML patients (13 female and 10 male) and seven samples of healthy volunteers using ELISA (Invitrogen). Twenty-two patients received induction chemotherapy regimen of cytarabine (100–200 mg/m2 for seven days) and daunorubicin (60 mg/m2 for three days), then consolidation chemotherapy with intermediate or high dose cytarabine. A total of 68 peripheral blood and bone marrow samples were included with a median age of 47 years (IQR: 23–57). At diagnosis, the median hemoglobin, white blood cells, and platelet counts were 8.0 g/dL (IQR: 6.0–9.0), 56 × 109/L (IQR: 36–68), and 33 × 109/L (IQR: 8–53), respectively. The median blast percentage was 50.0% (IQR: 26.0–7.07) in peripheral blood and 64.0% (IQR: 53.0–83.0) in bone marrow.
The expression of JAK1 and pJAK1 was analyzed in nine AML patients (five male and four female) and seven healthy donors. The median JAK1 expression in the peripheral blood was 20 (IQR: 9–51) at diagnosis and 31.5 (IQR: 4.0–77.0) in remission (p = 0.781), while in the bone marrow, it was 61.5 (IQR: 15.0–101.0) at diagnosis and 36 (IQR: 17–73) in remission (p = 0.694) [Figure 1a]. The overexpression percentage of JAK1 was 62.0% in peripheral blood at diagnosis and 66.0% at remission (p = 0.968), and in bone marrow, it was 83.0% at diagnosis and 62.0% in remission (p = 0.919).
In addition, the median pJAK1 expression in the peripheral blood was 14 (IQR: 6–25) at diagnosis and 58 (IQR: 4–108) in remission (p = 0.257), while in the bone marrow it was 14 (IQR: 1–78) at diagnosis and 32 (IQR: 5 – 55) in remission (p = 0.507) [Figure 1b]. The overexpression percentage of pJAK1 was 55.0% in peripheral blood and 83.0% in remission (p = 0.984) and it was 50.0% in bone marrow at diagnosis and 62.0% in remission (p = 0.997).
The expression of pJAK2 was analyzed in 19 AML patients (10 male and nine female) and seven healthy donors. We found that pJAK2 has lower expression levels in newly diagnosed AML patients compared to those in remission. In the peripheral blood, the median pJAK2 expression was 1 (IQR: 1–2) at diagnosis and 1 (IQR: 1–2) at remission (p > 0.999) whereas in bone marrow, it was 1 (IQR: 1–1) at diagnosis and 1 (IQR: 1– 3) at remission (p = 0.236) [Figure 1c]. In peripheral blood, the overexpression percentage of pJAK2 at diagnosis was 42.1% compared to 70.6% in remission (p = 0.054). In bone marrow, the overexpression of pJAK2 was 33.3% at diagnosis compared to 63.2% in remission (p = 0.148).
The expression of pSTAT3 was analyzed in 17 AML patients (nine male and eight female) and seven healthy donors. The pSTAT3 expression was lower at diagnosis compared to remission. In the peripheral blood, the median pSTAT3 expression was 4.5 (IQR: 1.0–9.0) at diagnosis and 1 (IQR: 1–1) in remission (p = 0.958) whereas in bone marrow, it was 1.5 (IQR: 1.0–9.0) at diagnosis and 1 (IQR: 1–1) in remission (p = 0.392) [Figure 1d]. In peripheral blood, the overexpression percentage of pSTAT3 at diagnosis was 31.6% compared to 5.3% in remission (p = 0.619). In bone marrow, the overexpression of pSTAT3 was 15.8% at diagnosis compared to 5.3% in remission (p = 0.868).
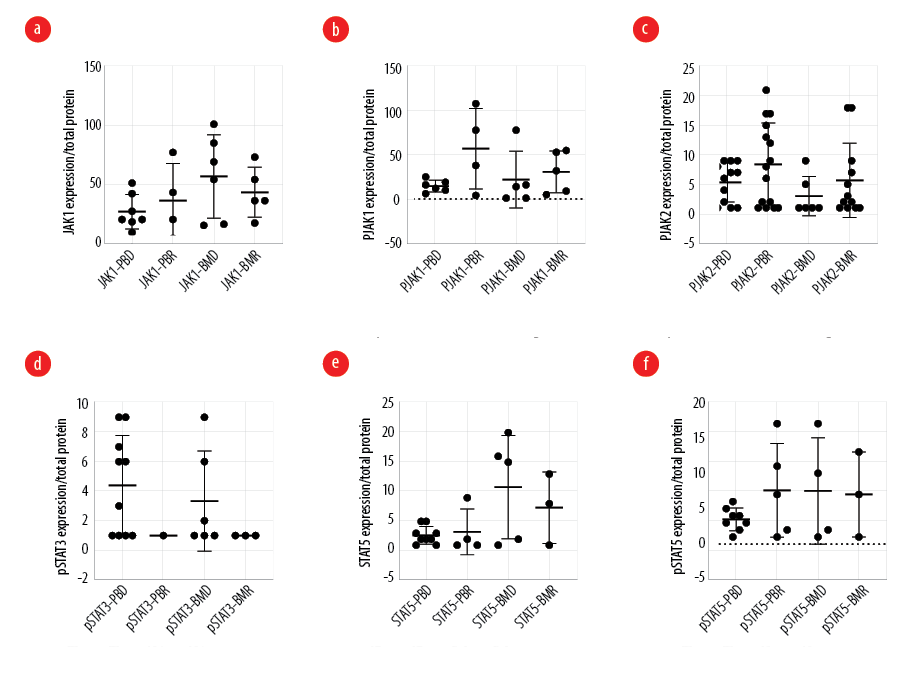 Figure 1: Protein expression of (a) JAK1 (b) pJAK1, (c) pJAK2, (d) pSTAT3, (e) STAT5, (f) pSTAT5 in peripheral blood and bone marrow of acute myeloid leukemia at diagnosis and remission.
Figure 1: Protein expression of (a) JAK1 (b) pJAK1, (c) pJAK2, (d) pSTAT3, (e) STAT5, (f) pSTAT5 in peripheral blood and bone marrow of acute myeloid leukemia at diagnosis and remission.
The expressions of STAT5 and pSTAT5 were analyzed in 17 AML patients and eight healthy donors. In the peripheral blood, the median STAT5 expression was 3 (IQR: 1–42) at diagnosis and 13.5 (IQR: 1.0–82.0) at remission (p = 0.273) whereas in bone marrow, it was 15 (IQR: 1–19) at diagnosis and 13 (IQR: 1–79) in remission (p = 0.746) [Figure 1e]. In peripheral blood, the overexpression percentage of STAT5 at diagnosis was 64.3% compared to 77.8% in remission (p = 0.994). In bone marrow, the overexpression of STAT5 was 50.0% compared to remission, which was 37.5% (p = 0.992).
In the peripheral blood, the median pSTAT5 expression was 3.5 (IQR: 1.0–6.0) at diagnosis and 7 (IQR: 1 – 17) in remission (p = 0.420) whereas in bone marrow, it was 6 (IQR: 1–17) at diagnosis and 7 (IQR: 1–13) in remission (p = 0.971) [Figure 1f]. In peripheral blood, the overexpression percentage of pSTAT5 at diagnosis was 50.0% compared to 66.0% in remission (p = 0.859). In bone marrow, the overexpression of pSTAT5 was 66.0% compared to remission, which was 50.0% in remission (p = 0.841).
The level of expression of all tested proteins (pJAK2, pJAK1, JAK1, pSTAT3, pSTAT5 and STAT5) did not impact the OS. The groups were divided based on the median expression of the protein. The hazard ratio and the p-values of the tested comparisons are listed in Table 1. The lower median survival rate in pJAK1 expression at peripheral blood was 83.0 weeks (HR = 0.4174; p = 0.452). In bone marrow, the lower median survival rate was 83.5 weeks (p = 1.000). In pJAK2 expression at peripheral blood, the lower median survival rate was 83.0 weeks, while the higher median survival rate was 86.0 (HR = 0.3724; p = 0.242) [Figure 2a]. In bone marrow, the lower median survival rate was 61.8 weeks (HR= 0.4293; p = 0.467) [Figure 2b]. In pSTAT3 expression at peripheral blood, the lower median survival rate was 83.5 weeks, while the higher median survival rate was 34.0 weeks (HR = 2.6847; p = 0.184) [Figure 2c]. In bone marrow, the lower median survival rate was 81.7 weeks, while the higher median survival rate was 34 weeks (HR = 1.4622; p = 0.758) [Figure 2d]. In STAT5 expression at peripheral blood, the lower median survival rate was 83.0 weeks, while the higher median survival rate was 86 weeks (HR = 0.6308; p = 0.620) [Figure 2, e and f]. The higher median survival rate in pSTAT5 expression was 83.0 weeks (HR = 0.9100; p = 0.925) [Figure 2g], while the lower median survival rate in bone marrow was 40.1 weeks (p = 1.000) [Figure 2h].
Table 1: The difference of overall survival (OS) rate based on the median survival rate.
|
pJAK1
|
PB
|
83.0
|
NA
|
0.4174
|
0.452
|
|
BM
|
83.5
|
NA
|
NA
|
1.000
|
|
pJAK2
|
PB
|
83.0
|
86.0
|
0.3724
|
0.242
|
|
BM
|
61.8
|
NA
|
0.4293
|
0.467
|
|
pSTAT3
|
PB
|
83.5
|
34.0
|
2.6847
|
0.184
|
|
BM
|
81.7
|
34.0
|
1.4622
|
0.758
|
|
STAT5
|
PB
|
83.0
|
86.0
|
0.6308
|
0.620
|
|
BM
|
NA
|
NA
|
NA
|
1.000
|
|
pSTAT5
|
PB
|
NA
|
83.0
|
0.9100
|
0.925
|
PB: peripheral blood; BM: bone marrow; HR: hazard ratio; NA: not applicable.
The estimate couldn't be calculated from variable results due to missing values.
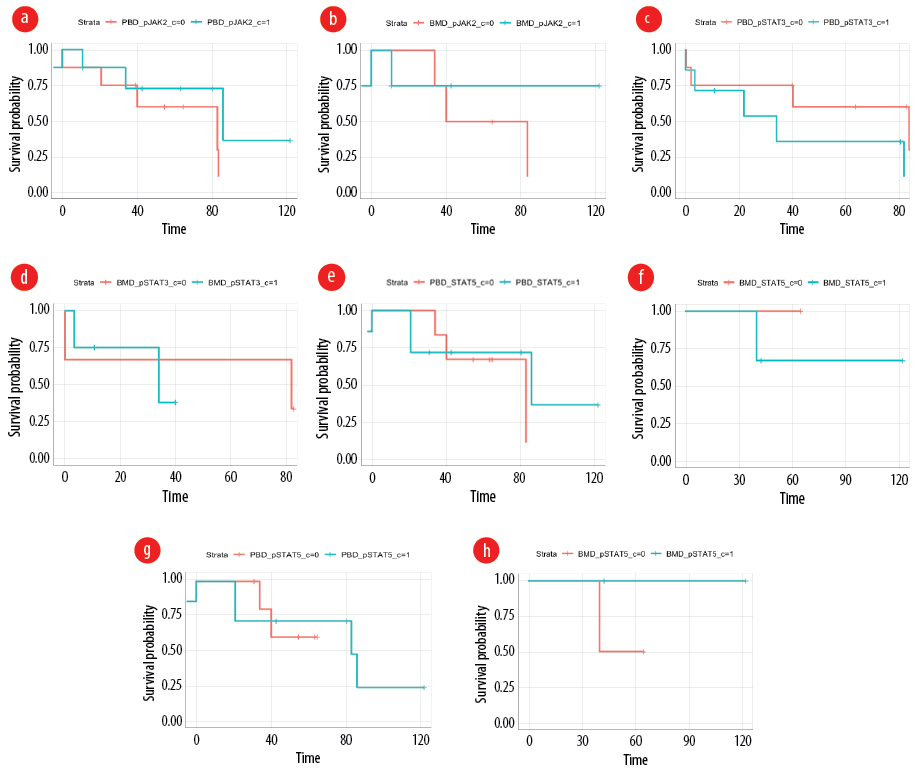 Figure 2: Overall survival of acute myeloid leukemia patients based on (a) pJAK2 expression levels in peripheral blood, (b) pJAK2 expression levels in bone marrow, (c) pSTAT3 expression levels in peripheral blood, (d) pSTAT3 expression levels in bone marrow, (e) STAT5 expression levels in peripheral blood, (f) STAT5 expression levels in bone marrow, (g) pSTAT5 expression levels in peripheral blood, and (h) OS of AML patients based on pSTAT5 expression levels in bone marrow.
Figure 2: Overall survival of acute myeloid leukemia patients based on (a) pJAK2 expression levels in peripheral blood, (b) pJAK2 expression levels in bone marrow, (c) pSTAT3 expression levels in peripheral blood, (d) pSTAT3 expression levels in bone marrow, (e) STAT5 expression levels in peripheral blood, (f) STAT5 expression levels in bone marrow, (g) pSTAT5 expression levels in peripheral blood, and (h) OS of AML patients based on pSTAT5 expression levels in bone marrow.
Discussion
At both the diagnostic and remission stages of AML, our research found no statistically significant change in the expression levels of the proteins JAK1, pJAK1, pJAK2, pSTAT3, STAT5, and pSTAT5 between bone marrow and peripheral blood samples. Changes in protein expression may have prognostic importance, and the JAK/STAT pathway is often dysregulated in AML. However, our results disprove this notion. We found no evidence that JAK/STAT proteins significantly affected AML patients' OS rates. Our investigation did not find any significant difference in the expression levels of pJAK2 in either peripheral blood or bone marrow between the diagnosis and remission. New evidence suggests elevated levels of activated pJAK2 in bone marrow samples taken from a large cohort of AML patients (n = 77). According to a recent study by Ikezoe et al,4 pJAK2 expression predicts clinical outcomes and may be a molecular target of AML. Patients with de novo or secondary AML were found to have an elevated white blood cell count, decreased platelet count, and worse survival rate when pJAK2 levels were higher. Although there were greater levels of pJAK2, the JAK2 V617F mutation was found in only one of the 77 patients with AML. This provides further evidence that distinct mechanisms contribute to JAK/STAT pathway dysregulation. Cook et al,10 found that individuals with AML had a poorer prognosis and shorter OS when their expression levels of pJAK2 were higher. While the degree of activation varied between samples, it was discovered that pJAK2 levels were active (100%).11 This was seen in bone marrow samples from AML patients. In line with other studies, this one also found no significant connection between pJAK1 expression levels at diagnosis and OS in either peripheral blood or bone marrow samples.9 The HR was 5.21 (95% CI: 1.212–4.041; p = 0.023), although prior studies shown that high levels of pJAK2 are still a strong predictor of OS.4 Researchers also found that pJAK2 expression was associated with decreased platelet counts, increased white blood cell counts, and shorter survival durations in a large cohort of AML patients (n = 77) using bone marrow samples.4 Patients with AML who expressed JAK2 had a worse prognosis than those with STAT5 expression, according to another study.12 As shown by reduced OS and complete remission rates, high pJAK2 levels were significantly associated with a worse clinical outcome.8 According to research conducted by Birkenkamp et al,13 a significant number of AML patients (44–76%) exhibited constitutive activation of STAT3 and STAT5. Interestingly, the disease-free survival rate was worse for those with constitutive STAT3 activity compared to those without. AML blasts with constitutive activation of the STAT3 protein had a low disease-free life.8 Compared to remission-free individuals, our study showed that AML patients exhibited lower expression levels of pSTAT3. This new finding by Short et al,14 is in line with previous studies that have shown pSTAT3's role in leukemia cell viability and proliferation.Some studies have shown that pSTAT3 may improve the prognosis for leukemia. An increase in STAT3 phosphorylation at Y705 and S727 in response to cytokine stimulation has been linked to better disease-free and survival rates in AML patients.15 According to research conducted by Khoury et al,16 an improvement in OS was linked to positive tyrosine phosphorylation of STAT3. Also, compared to the first diagnosis, recurrence was associated with higher levels of STAT3B isoform expression.17 Standard AML therapy was associated with a worse prognosis for individuals whose STAT3 and STAT5 phosphorylation was amplified after growth factor stimulation, according to a few investigations.18 Additionally, in some individuals (53%), STAT5 was shown to be activated in newly isolated CD34+ AML cells.19 Investigations have shown that AML cell lines from humans have STAT transcription factors that are constitutively active.20 Constitutive STAT3 activation has been associated in the past with a decreased disease-free survival rate in AML.8 An analysis of AML patients conducted in 2011 indicated that OS was unaffected by high constitutive pSTAT3 expression.8 Even after attaining complete remission, pSTAT5 expression in freshly diagnosed AML blasts is linked to worse overall mortality, progression-free survival, and recurrence likelihood.10 Patients with newly diagnosed AML who express pSTAT5 in their blasts have a greater chance of recurrence after establishing complete remission, poor OS, and progression-free survival.21 Enhanced STAT5 activity has been linked to FLT3 activation, FLT3 mutations, according to prior studies. Research done by Obermann et al,22 reported the FLT3-ITD mutation was associated with pSTAT5 expression levels higher than 1%.
Researchers have shown that newly diagnosed AML patients and those in remission have similar levels of JAK2, STAT3, and STAT5 expression. Such evidence points to a potential interaction between these proteins that facilitates leukemogenesis and the development of illness.23
Benekli et al,8 discovered that constitutive STAT3 activation in AML affected disease-free survival but had no detrimental effect on OS. According to certain research, patients with AML had better disease-free and survival rates when the phosphorylation level of STAT3 on Y705 and S727 rose in response to cytokine stimulation.24 Findings from Levidou et al,25 suggested a weak association between tyrosine phosphorylated STAT3 and OS time.
The expression of pSTAT5 in bone marrow from 112 individuals with newly diagnosed AML was also studied, and the results showed that STAT5 activation was significantly associated with poor OS and delayed progression.21 It is possible that the limited sample size does not adequately reflect the whole AML population, which is one of the limitations that our research recognize. Although a bigger and more representative sample would have allowed for more robust statistical analysis, our small sample size may have compromised its capacity to detect meaningful changes. The prognostic implications of the expression of proteins in the JAK/STAT pathway and other key factors such as genetic mutations and cytogenetic abnormalities were not considered.
There needs to be further research on JAK/STAT protein dysregulation in AML to clarify its causes. The possible effects of mutations in additional JAK/STAT pathway genes on AML etiology and OS results should also be investigated.
Conclusion
AML patients have overexpressed JAK/STAT proteins at the time of diagnosis in both their peripheral blood and bone marrow. However, neither in peripheral blood nor bone marrow, was there a significant difference in the expression levels of these proteins between the diagnosis and remission stages. It is important to note that the limited sample size of the study and the natural heterogeneity of AML may be responsible for the lack of a statistically meaningful difference.
Additionally, the levels of pSTAT3, pSTAT5/STAT5, pJAK2, pJAK1, and JAK1 expression in bone marrow or peripheral blood did not significantly affect the OS of AML patients. This shows that factors other than the levels of JAK/STAT protein expression may be more important in predicting the prognosis for AML patients.
The study’s limitations, such as the limited sample size, potential differences in the AML subtype, and patient characteristics, must be considered. To acquire a more thorough grasp of these findings, it is also advised to consider the larger body of research on AML.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Abelson S, Collord G, Ng SW, Weissbrod O, Mendelson Cohen N, Niemeyer E, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 2018 Jul;559(7714):400-404.
- 2. Raivola J, Haikarainen T, Abraham BG, Silvennoinen O. Janus kinases in leukemia. Cancers (Basel) 2021 Feb;13(4):800.
- 3. Schuringa JJ, Wierenga AT, Kruijer W, Vellenga E. Constitutive Stat3, Tyr705, and Ser727 phosphorylation in acute myeloid leukemia cells caused by the autocrine secretion of interleukin-6. Blood 2000 Jun;95(12):3765-3770.
- 4. Ikezoe T, Kojima S, Furihata M, Yang J, Nishioka C, Takeuchi A, et al. Expression of p-JAK2 predicts clinical outcome and is a potential molecular target of acute myelogenous leukemia. Int J Cancer 2011 Nov;129(10):2512-2521.
- 5. Venugopal S, Bar-Natan M, Mascarenhas JO. JAKs to STATs: A tantalizing therapeutic target in acute myeloid leukemia. Blood Rev 2020 Mar;40:100634.
- 6. Brachet-Botineau M, Polomski M, Neubauer HA, Juen L, Hédou D, Viaud-Massuard MC, et al. Pharmacological inhibition of oncogenic STAT3 and STAT5 signaling in hematopoietic cancers. Cancers (Basel) 2020 Jan;12(1):240.
- 7. Blink M, Buitenkamp TD, van den Heuvel-Eibrink MM, Danen-van Oorschot AA, de Haas V, Reinhardt D, et al. Frequency and prognostic implications of JAK 1-3 aberrations in Down syndrome acute lymphoblastic and myeloid leukemia. Leukemia 2011 Aug;25(8):1365-1368.
- 8. Benekli M, Xia Z, Donohue KA, Ford LA, Pixley LA, Baer MR, et al. Constitutive activity of signal transducer and activator of transcription 3 protein in acute myeloid leukemia blasts is associated with short disease-free survival. Blood 2002 Jan;99(1):252-257.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 1968;97:77-89.
- 10. Cook AM, Li L, Ho Y, Lin A, Li L, Stein A, et al. Role of altered growth factor receptor-mediated JAK2 signaling in growth and maintenance of human acute myeloid leukemia stem cells. Blood 2014 May;123(18):2826-2837.
- 11. Steensma DP, McClure RF, Karp JE, Tefferi A, Lasho TL, Powell HL, et al. JAK2 V617F is a rare finding in de novo acute myeloid leukemia, but STAT3 activation is common and remains unexplained. Leukemia 2006 Jun;20(6):971-978.
- 12. Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene 2013 May;32(21):2601-2613.
- 14. Birkenkamp KU, Geugien M, Lemmink HH, Kruijer W, Vellenga E. Regulation of constitutive STAT5 phosphorylation in acute myeloid leukemia blasts. Leukemia 2001 Dec;15(12):1923-1931.
- 15. Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet 2018 Aug;392(10147):593-606.
- 16. Vella A, D’Aversa E, Api M, Breveglieri G, Allegri M, Giacomazzi A, et al. mTOR and STAT3 pathway hyper-activation is associated with elevated interleukin-6 levels in patients with shwachman-diamond syndrome: further evidence of lymphoid lineage impairment. Cancers 2020 Mar 5;12(3):597.
- 17. Khoury JD, Medeiros LJ, Rassidakis GZ, Yared MA, Tsioli P, Leventaki V, et al. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK- anaplastic large cell lymphoma. Clin Cancer Res 2003 Sep;9(10 Pt 1):3692-3699.
- 18. Xia Z, Sait SN, Baer MR, Barcos M, Donohue KA, Lawrence D, et al. Truncated STAT proteins are prevalent at relapse of acute myeloid leukemia. Leuk Res 2001 Jun;25(6):473-482.
- 19. Kornblau SM, Minden MD, Rosen DB, Putta S, Cohen A, Covey T, et al. Dynamic single-cell network profiles in acute myelogenous leukemia are associated with patient response to standard induction therapy. Clin Cancer Res 2010 Jul;16(14):3721-3733.
- 20. Blackburn LM, Bender S, Brown S. Acute leukemia: diagnosis and treatment. Semin Oncol Nurs 2019 Dec;35(6):150950.
- 21. Spiekermann K, Biethahn S, Wilde S, Hiddemann W, Alves F. Constitutive activation of STAT transcription factors in acute myelogenous leukemia. Eur J Haematol 2001 Aug;67(2):63-71.
- 22. Brady A, Gibson S, Rybicki L, Hsi E, Saunthararajah Y, Sekeres MA, et al. Expression of phosphorylated signal transducer and activator of transcription 5 is associated with an increased risk of death in acute myeloid leukemia. Eur J Haematol 2012 Oct;89(4):288-293.
- 23. Obermann EC, Arber C, Jotterand M, Tichelli A, Hirschmann P, Tzankov A. Expression of pSTAT5 predicts FLT3 internal tandem duplications in acute myeloid leukemia. Ann Hematol 2010 Jul;89(7):663-669.
- 24. De Kouchkovsky I, Abdul-Hay M. ‘Acute myeloid leukemia: a comprehensive review and 2016 update’. Blood Cancer J 2016 Jul;6(7):e441.
- 25. Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB, Tweardy DJ. Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood 2011 May;117(21):5701-5709.
- 26. Levidou G, Sachanas S, Pangalis GA, Kalpadakis C, Yiakoumis X, Moschogiannis M, et al. Immunohistochemical analysis of IL-6, IL-8/CXCR2 axis, Tyr p-STAT-3, and SOCS-3 in lymph nodes from patients with chronic lymphocytic leukemia: correlation between microvascular characteristics and prognostic significance. Biomed Res Int 2014;2014:251479.