|
Abstract
Chronic pulmonary artery narrowing may result from diverse causes; congenital as well as acquired. The relative hypoperfusion of the lung results in hypertrophy of multiple systemic arteries. Such patients can present with recurrent hemoptysis from hypertrophied systemic arteries, most commonly bronchial arteries. These patients remain undiagnosed for a long time because of the lack of awareness of this entity. We present three cases of chronic pulmonary artery narrowing presenting with massive hemoptysis.
Keywords: Hemoptysis; Pulmonary artery narrowing; Tuberculosis.
Introduction
Several factors are responsible for pulmonary artery narrowing, with most cases in the developing world being secondary to infection. Tuberculosis can lead to pulmonary artery compression by causing mediastinal lymphadenopathy or mediastinal fibrosis. The latter presentation can also be related to histoplasmosis in endemic foci. These as well as several other less frequent conditions can cause pulmonary hypoperfusion and compensatory bronchial over circulation with bronchial artery hypertrophy. This places the patient at risk for hemorrhage and hemoptysis, which can sometimes be life threatening. Awareness of the pathogenesis of hemoptysis will provide an opportunity to treat patients with multiple available modalities depending on the patient and the underlying disease profile. We attempt to highlight the importance of early recognition and potential treatment options for this less understood condition.
Case 1
A 12-year-old male had cough, low grade fever, weight loss and recurrent hemoptysis for three years. This time, he presented to the emergency department with massive hemoptysis. He had received a course of antitubercular treatment (ATT), based on clinical suspicion, though the sputum examination was negative. Examination revealed severe pallor, bilateral digital clubbing and bronchial breathing on the right side. The cardiovascular system examination was normal. Chest X-ray (CXR) showed opaque right hemithorax (Fig. 1a). Contrast enhanced computed tomography (CECT) of chest showed collapse-consolidation of right middle and lower lobe (Fig. 1b). The patient was started on ATT and a CXR was repeated after 1 month, which showed dramatic response with complete resolution of collapse-consolidation (Fig. 1c). Two months later, the patient again presented with a severe bout of hemoptysis and a CXR revealed almost complete opaque right hemithorax (not shown here). CECT chest performed seven days later showed narrowing of the right pulmonary artery caused by mass effect of a large necrotic hilar lymph node with partial aeration of the lung by now (Fig. 1d). As the hemoptysis was recurrent and severe, embolization was planned. Flush aortogram at the level of the arch of the aorta revealed hypertrophied bilateral bronchial, right intercosto-bronchial, right internal mammary and right inferior phrenic artery (Figs. 1e and f). These arteries were embolized using PVA particles in two sittings. The patient suffered no hemoptysis for the follow-up period (two years). Follow-up CT revealed resolution of nodes with well aerated lung. The recurrent collapse at presentation was likely due to clot impaction in the right main and intermediate bronchus.
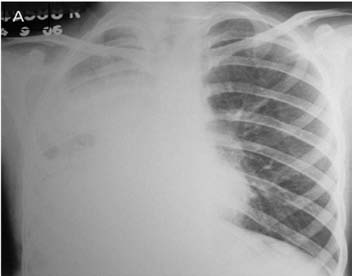
Figure 1a: Frontal chest radiograph showing nearly opaque right hemithorax with mild ipsilateral mediastinal shift.
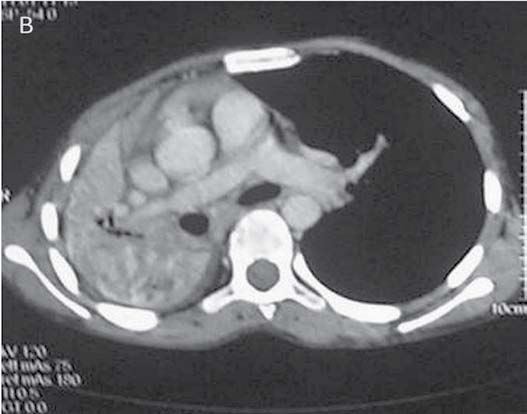
Figure 1b: CECT chest done on the same day as CXR revealed collapse-consolidation of right middle and lower lobes.
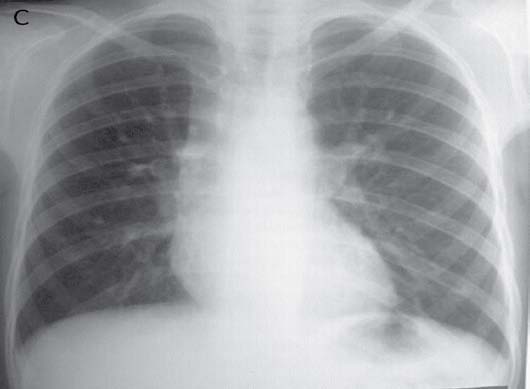
Figure 1c: Repeat CXR following the start of ATTshows showing marked resolution of collapse-consolidation.
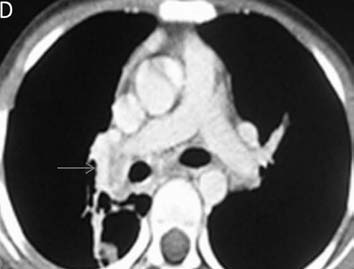
Figure 1d: CTPA performed during the episode of hemoptysis revealed compression of the right pulmonary artery (arrow) at the level of right hilum by a bulky necrotic hilar lymph node.
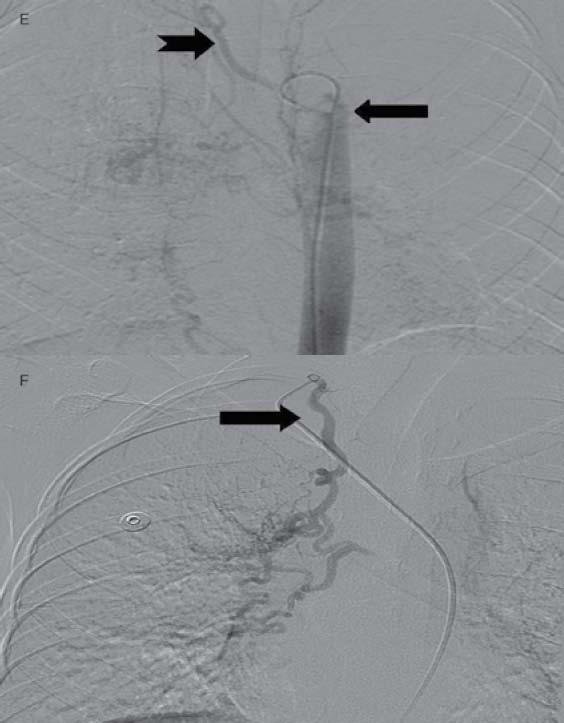
Figures 1e and f: Images from DSA for embolization of the systemic feeders (mandated by recurrent massive hemoptysis) showing hypertrophied bilateral bronchial (arrow, Fig 1e) right intercosto-bronchial (notched arrow, Fig. 1e), right internal mammary (arrow, Fig 1f) and right inferior phrenic (not shown here) arteries which were embolized using PVA in two sittings.
Case 2
A 38-year-old female presented with chief complaint of on-and-off hemoptysis since 16 years. There was associated history of weight loss and intake of ATT thrice (by private practitioner, based on hemoptysis and mediastinal widening on chest X-ray), with last course of ATT completed one year back. The patient had severe pallor and crepitation on the right side. The rest of the respiratory and cardiovascular system examination was normal. CXR showed volume loss on the right side with ipsilateral mediastinal shift. (Fig. 2a)
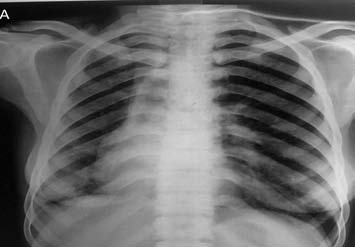
Figure 2a: Frontal chest radiograph showing volume loss on the right side with ipsilateral mediastinal shift.
CT pulmonary angiography (CTPA) showed a fibrotic mass-like lesion encasing the mediastinal vessels causing narrowing of the right pulmonary artery (Fig. 2b). A hypertrophied bronchial artery was also noted on the right side. The patient was posted for bronchial artery embolization (BAE). Flush aortic angiogram revealed a hypertrophied right bronchial artery. Selective cannulation and embolization of this artery was done using polyvinyl alcohol (PVA) 300-500 µ particles (Fig. 2c). The patient experienced significant improvement in the frequency and amount of hemoptysis.
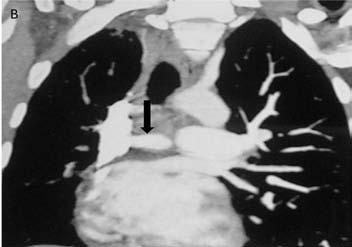
Figure 2b: CT pulmonary angiography (CTPA) of the same patient done a few days later shows a fibrotic mass like lesion encasing mediastinal vessels. In particular, there is significant narrowing of right pulmonary artery (arrow). A hypertrophied bronchial artery is also noted on the same side.
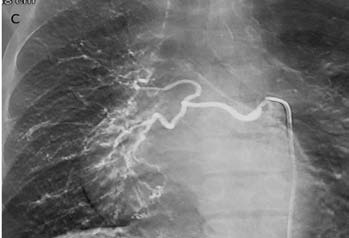
Figure 2c: DSA image shows hypertrophied right bronchial artery as expected from CTPA. This was successfully embolized using PVA.
Case 3
A 13-year-old female presented with five bouts of massive hemoptysis since two years. The patient had completed ATT six months back based on the fine needle aspiration of cervical lymph nodes which was suggestive of tuberculosis. The present bout of hemoptysis mandated hospitalization and a request for BAE was received. Review of the patient’s investigations was done. CXR obtained one month back revealed widening of right paratracheal stripe with right lung volume loss (Fig. 3a).
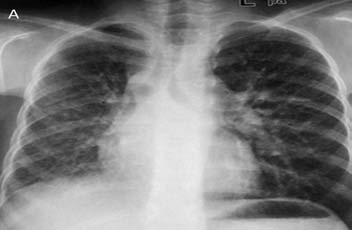
Figure 3a: Frontal chest radiograph reveals widening of the right paratracheal stripe with right lung volume loss and subtle reticular opacities.
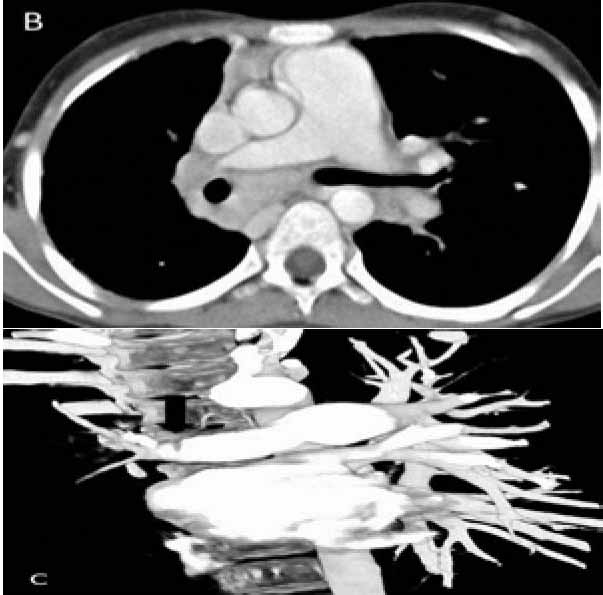
Figure 3b and c: CTPA (axial and coronal volume rendered image) shows mediastinal lymphadenopathy encasing the right pulmonary artery with resultant narrowing (arrow).
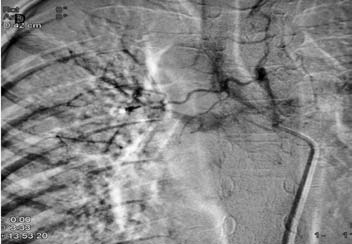
Figure 3d: DSA image shows hypertrophied right bronchial artery with pulmonary arterial shunting. Embolization of this artery was achieved with PVA.
CTPA that was done at the same time as the CXR showed mediastinal lymphadenopathy encasing the right pulmonary artery and resultant narrowing. No systemic collaterals could be seen on this study (Figs. 3b and 3c). However, flush aortic angiogram did reveal hypertrophied right bronchial artery with pulmonary arterial shunting (Fig. 3d). Embolization of the artery was achieved with PVA particles 500 µ. The patient had a temporary relief for two months; but she later suffered a massive bout of hemoptysis to which she succumbed.
Discussion
Chronic pulmonary artery narrowing may result from diverse causes like congenital, infectious, inflammatory and chronic thromboembolism.1 Infectious causes account for the majority of cases in our setting. This is due to a high prevalence of tuberculosis in India. Narrowing of pulmonary artery in the setting of thoracic tuberculosis may result from mediastinal lymphadenopathy or mediastinal fibrosis. Mediastinal fibrosis is a benign chronic inflammation leading to the proliferation of fibrous tissue within the mediastinum.2 It may be focal or diffuse. Focal mediastinal fibrosis presents as a calcified soft tissue mass in the paratracheal and hilar location. It occurs secondary to tuberculosis (in the developing world) and histoplasmosis (in United States).3 The diffuse form produces an infiltrative non-calcified soft tissue involving more than one mediastinal compartment. It occurs in association with various autoimmune disorders, drugs, retroperitoneal fibrosis; few cases are idiopathic.3 Narrowing most commonly involves the superior vena cava and central airways; while narrowing of a pulmonary artery is much less common.3,4 Unilateral or bilateral involvement may result but the right pulmonary artery is most commonly involved1 (as noted in our series of patients). Similarly, hilar lymphadenopathy may cause narrowing of the ipsilateral pulmonary artery.
Pulmonary artery narrowing leads to pulmonary hypoperfusion and resultant takeover by the systemic circulation in the form of bronchial artery hypertrophy. Once systemic arteries hypertrophy, patients present with hemoptysis of varying severity. Similar changes are also observed in patients with chronic pulmonary thromboembolism which although reported, the literature available is scanty.5 Pulmonary artery narrowing secondary to nodal disease or fibrosing mediastinitis with resultant bronchial artery hypertrophy is either not identified or is under reported. Thus, awareness of this entity may lead to early diagnosis and management of these patients.
CTPA is the investigation of choice to demonstrate pulmonary artery narrowing. It also demonstrates hypertrophied bronchial or other systemic arteries and hence provides an important road map for angiography.6 Digital subtraction angiography (DSA) is rarely used for diagnosis nowadays; it is mainly reserved for endovascular management. There are various therapeutic options for such patients including medical therapy, DSA and embolization of hypertrophied systemic arteries, as well as surgical treatment.3 Medical therapy consists of ATT or systemic antifungal agents and corticosteroids. At angiography, a flush aortogram is obtained at a level just below the arch of the aorta to demonstrate hypertrophied bronchial arteries. Cannulation of the hypertrophied arteries is also done based on the road map provided by CTPA. Embolization is done using PVA particles. Particle size most commonly used is 300-500 µ. A staged procedure involving multiple sittings may be required to avoid pulmonary infarction. Surgical resection of the affected tissues may result in cure or amelioration of the signs and symptoms.7 However, a complete resection often requires extensive airway or vascular reconstruction.8 These are technically challenging procedures available only at a few centres and have a high morbidity and mortality.9
Conclusion
Due to high prevalence of tubercular nodes and resulting fibrosing mediastinitis in the developing part of the world, the importance and awareness of treatment of this albeit uncommon but not rare condition is emphasized. Early diagnosis and endovascular management can be life-saving in an otherwise under-reported and potentially catastrophic entity.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Castañer E, Gallardo X, Rimola J, Pallardó Y, Mata JM, Perendreu J, et al. Congenital and acquired pulmonary artery anomalies in the adult: radiologic overview. Radiographics 2006 Mar-Apr;26(2):349-371.
2. Sherrick AD, Brown LR, Harms GF, Myers JL. The radiographic findings of fibrosing mediastinitis. Chest 1994 Aug;106(2):484-489.
3. Rossi SE, McAdams HP, Rosado-de-Christenson ML, Franks TJ, Galvin JR. Fibrosing mediastinitis. Radiographics 2001 May-Jun;21(3):737-757.
4. Berry DF, Buccigrossi D, Peabody J, Peterson KL, Moser KM. Pulmonary vascular occlusion and fibrosing mediastinitis. Chest 1986 Feb;89(2):296-301.
5. Reesink HJ, van Delden OM, Kloek JJ, Jansen HM, Reekers JA, Bresser P. Embolization for hemoptysis in chronic thromboembolic pulmonary hypertension: report of two cases and a review of the literature. Cardiovasc Intervent Radiol 2007 Jan-Feb;30(1):136-139.
6. Khalil A, Parrot A, Nedelcu C, Fartoukh M, Marsault C, Carette MF. Severe hemoptysis of pulmonary arterial origin: signs and role of multidetector row CT angiography. Chest 2008 Jan;133(1):212-219.
7. Dunn EJ, Ulicny KS Jr, Wright CB, Gottesman L. Surgical implications of sclerosing mediastinitis. A report of six cases and review of the literature. Chest 1990 Feb;97(2):338-346.
8. Smith JS, Kadiev S, Diaz P, Cheatham J. Pulmonary artery stenosis secondary to fibrosing mediastinitis: management with cutting balloon angioplasty and endovascular stenting. Vasc Endovascular Surg 2011 Feb;45(2):170-173.
9. Loyd JE, Tillman BF, Atkinson JB, Des Prez RM. Mediastinal fibrosis complicating histoplasmosis. Medicine (Baltimore) 1988 Sep;67(5):295-310.
|