Pulmonary embolism (PE) is the third most common acute cardiovascular disease that can lead to death after myocardial infarctions and stroke.1 The yearly rate of incidence of PE is estimated at 39–115 per 100 000 population.2 In the recent years, acute PE fatality rates have been declining in developed countries but rising in developing countries.2
Both genetic and environmental factors may participate in the development of PE.3 The risk factors for PE include a hypercoagulable state where there is a stasis of blood such as in pregnancy, surgery, immobilization, traveling, and malignancy.3 PE is often asymptomatic and discovered incidentally during investigations for another disease.4 Patients can also present with symptoms and signs that are vague and non-specific.5
Toll-like receptors (TLRs), especially TLR2 and TLR4, play an important role in the host defense mechanism against microorganisms.6 The expression of TLR2, TLR4, and interleukin-1 beta (IL-1β) tend to be higher in patients with PE.7 Other ILs such as IL-10 are considered to be immune-regulatory cytokines with anti-tumor effects.8 The level of IL-10 is significantly raised in patients having massive PE compared to patients with low-risk and sub-massive disease, reflecting the stronger inflammatory response to large embolisms.9 Circulating vascular cell adhesion molecule-1 is a cytokine-inducible endothelial cell adhesion molecule.10 Also, circulating vascular cell adhesion molecule-1 is
a platelet activation marker whose level significantly increases in response to PE.11
A paradoxical risk of PE exists in patients with immune thrombocytopenia, an autoimmune disease with high risk of bleeding and thrombocytopenia.12 Excessive use of glucocorticoids in immune thrombocytopenia patients to control bleeding can lead to a hypercoagulable state, raising the risk of PE which is not always clinically easy to prevent or even detect.13
Upon clinical suspicion of PE, diagnosis starts with clinical evaluation and laboratory markers such as D-dimer. In hemodynamically unstable patients with high-risk PE, bedside echocardiogram (echo) or emergency computed tomography pulmonary angiogram (CTPA) is recommended.14 Ventilation/perfusion scintigraphy and lower-limb ultrasound can be used to rule out deep vein thrombus. Other imaging modalities such as chest X-ray (CXR) are not specific to PE; but can be used to exclude other causes for dyspnea and chest pain.15 Electrocardiographic (ECG) findings such as S1Q3T3 can indicate a right ventricular (RV) strain that may be found in severe PE.16 However, in less severe cases, ECG might only show sinus tachycardia. Other predictors such as higher mean-platelet volume are associated with an immediate diagnosis of acute PE, and it can aslo predict serious infections such as spontaneous bacterial peritonitis, which is a major cause of mortality in patients with liver cirrhosis. 17,18
A study in Oman found that all three clinical prediction rules could help exclude PE in patients with malignancies.19 Another study found that Omani patients with sickle cell anemia who contracted PE were at high risk of morbidity and mortality.20 However, to our knowledge, no study has evaluated the clinical situation and adherence to guidelines for the management of PE among Omani patients. Therefore, this situational analysis aimed to provide a proper view of the predisposing factors, diagnostic issues, and adherence to the uptodate management strategies.
Methods
This retrospective cross-sectional situational analysis study was approved by the ethical committee of the Ministry of Health, Oman (ref: SRC#94/2021). All data related to acute pulmonary embolism cases were collected from the electronic medical record system of the Royal Hospital, a tertiary referral institution in Muscat.
We reviewed the medical files of all patients who were diagnosed with acute PE. Participants were selected based on the following criteria: the patient (1) is an Omani national, (2) was diagnosed with acute PE as confirmed by CTPA [Figure 1], (3) aged > 13 years at diagnosis, and (4) diagnosed at the Royal Hospital, during January 2010 to December 2021. All cases without CTPA images were excluded.
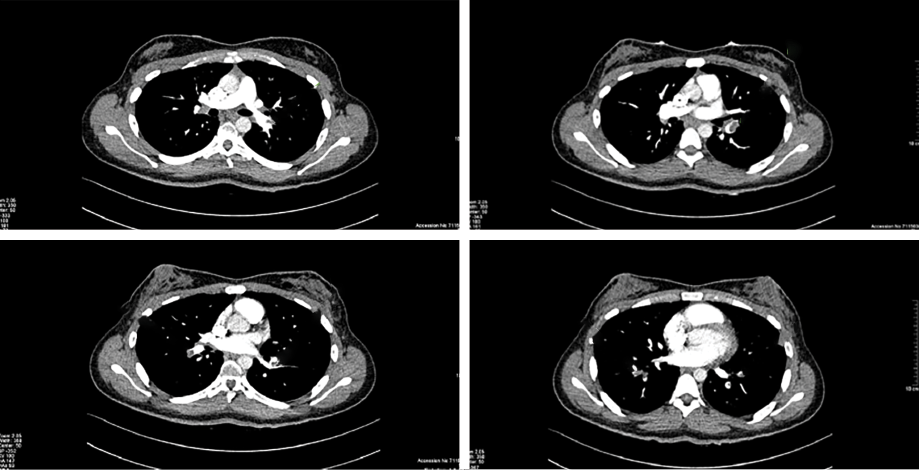 Figure 1: Typical computed tomography pulmonary angiogram images showing acute pulmonary embolism.
Figure 1: Typical computed tomography pulmonary angiogram images showing acute pulmonary embolism.
The data retrieved for the study included each patient’s demographic data, risk factors for PE, history of chronic conditions, symptoms of PE on presentation, laboratory parameters, imaging data including CTPA, ultrasound, and CXR, management details, and outcome including mortality associated with PE.
Comparative observational statistics were used to present the percentage of people with clinical data, laboratory parameters, and various comorbidities and outcomes. Data were described as frequencies and percentages of categorical variables. Continuous variables were reported as median and range or as mean and SD. Univariate comparisons between various lab findings were calculated using independent samples median tests. The data entry was rechecked by two researchers. All statistical calculations were performed using the STATA 16 software (version 16.0, STATA Inc, Chicago, Illinois, USA).
Results
During the study, 438 patients were proven to have PE on CTPA and were included in the analysis.
Males represented 223 (50.9%) of the study population. Most patients (278; 63.5%) were residents of Muscat governate and the rest were mostly from the nearby governorates. The mean age was 53.3±18.5 and the median age was 52.95. The oldest patient was aged 105 years and the youngest 15 years [Table 1].
Table 1: The demography of Omani patients with pulmonary embolism (N = 438).
|
Total patients, n (%)
|
438 (100)
|
215 (49.1)
|
223 (50.9)
|
|
|
Age in years, mean ± SD
|
53.3 ± 18.5
|
51.5 ± 18.3
|
55.0 ± 18.5
|
0.048
|
Table 2 shows clinical risk factors found in our participants. A quarter of all participants had to undergo surgery (109; 24.9%), which was found to be the main risk factor for morbidity and mortality. Malignancy was found in 73 (16.7%) patients. Pregnancy was reported at presentation by 21/215 (9.8%) female patients. Only 49 (11.2%) patients were bedbound, and 24 (5.5%) patients presented with trauma.
Table 2: The clinical risk factors found in Omani patients with pulmonary embolism (N = 438).
|
Number of patients (%)
|
438 (100)
|
215 (49.1)
|
223 (50.9)
|
|
|
BMI, mean ± SD
|
29.7 ± 7.3
|
30.9 ± 8.0
|
28.4 ± 6.3
|
0.005
|
|
Pregnancy
|
21 (4.8)
|
21 (4.8)
|
|
|
|
Malignancy
|
73 (16.7)
|
42 (9.6)
|
31 (7.7)
|
0.114
|
|
Trauma
|
24 (5.5)
|
11 (2.5)
|
13 (3.0)
|
0.743
|
|
Surgery
|
109 (24.9)
|
58 (13.2)
|
51 (11.6)
|
0.320
|
|
Bedbound
|
49 (11.2)
|
23 (5.3)
|
26 (5.9)
|
0.750
|
|
Travel
|
33 (7.5)
|
12 (2.7)
|
21 (4.8)
|
0.128
|
|
Smoking
|
29 (6.6)
|
1 (0.2)
|
28 (6.4)
|
< 0.001
|
|
Family history
|
5 (1.1)
|
1 (0.2)
|
4 (0.9)
|
0.191
|
|
SCD
|
16 (3.7)
|
10 (2.3)
|
6 (1.4)
|
0.457
|
|
DVT
|
72 (16.4)
|
33 (7.5)
|
39 (8.9)
|
0.546
|
|
COVID-19
|
48 (11.0)
|
19 (4.3)
|
29 (6.6)
|
0.163
|
|
DM
|
97 (22.1)
|
45 (10.3)
|
52 (11.9)
|
0.547
|
|
HTN
|
152 (34.7)
|
72 (16.4)
|
80 (18.3)
|
0.600
|
|
CLD
|
39 (8.9)
|
24 (5.5)
|
15 (3.4)
|
0.103
|
|
CLiD
|
9 (2.1)
|
1 (0.2)
|
8 (1.8)
|
0.021
|
|
No CKD
|
391 (89.3)
|
196 (44.7)
|
195 (44.5)
|
|
|
CKD all stages
|
|
|
|
0.035
|
|
CKD stage 1
|
2 (0.5)
|
2 (0.5)
|
0 (0.0)
|
|
|
CKD stage 2
|
1 (0.2)
|
1 (0.2)
|
0 (0.0)
|
|
|
CKD stage 3
|
6 (1.4)
|
0 (0.0)
|
6 (1.4)
|
|
|
CKD stage 4
|
3 (0.7)
|
0 (0.0)
|
3 (0.7)
|
|
BMI: body mass index; SCD: sickle cell disease; DVT: deep vein thrombus; DM: diabetes mellitus; HTN: hypertension; CLD: chronic lung disease; CLiD: chronic liver disease; CKD: chronic kidney disease.
The majority of our participants had vulnerabilities and diseases related to lifestyle such as high body mass index (BMI) (mean = 29.7±7.3 kg/m2), hypertension (HTN) (152; 34.7%), diabetes mellitus (DM) (97; 22.1%), chronic kidney disease (CKD) (46; 10.5%), and chronic liver disease (9; 2.1%). Regarding respiratory comorbidities, 48 (11.0%) patients had COVID-19 and 39 (8.9%) had lung disease. Despite the high consanguinity in Oman, genetic risk factors such as a family history of PE were found only in five (1.1%) patients and sickle cell disease in 16 (3.7%) patients.
Table 3 shows the various clinical presentation, where dyspnea was reported in 293 (66.9%). We found that 162 (37.0%) patients presented with chest pain. Cough was reported by 123 (28.1%) patients, while hemoptysis was reported in 18 (4.1%); 15 males and three females; p = 0.005.
Table 3: Clinical presentation signs and symptoms among Omani patients with pulmonary embolism
(N = 438).
|
Syncope
|
23 (5.3)
|
13 (3.0)
|
10 (2.3)
|
0.457
|
|
Dyspnea
|
293 (66.9)
|
150 (34.2)
|
143 (32.6)
|
0.185
|
|
Chest pain
|
162 (37.0)
|
76 (17.4)
|
86 (19.6)
|
0.509
|
|
Cough
|
123 (28.1)
|
55 (12.6)
|
68 (15.5)
|
0.265
|
|
Hemoptysis
|
18 (4.1)
|
3 (0.7)
|
15 (3.4)
|
0.005
|
A single patient may have had more than one symptom.
Table 4 shows the laboratory test results. Most patients had low mean hemoglobin (Hb) of 11.6±2.3. Mean platelet levels were also lower than normal at 282.4±144. Positive antiphospholipid level was higher in males than females. Unfortunately, protein S and protein C tests were conducted only in a small number of patients [Table 4].
Table 4: Laboratory test results among Omani patients with pulmonary embolism (N = 438).
|
Total patients, n (%)
|
438 (100)
|
215 (49.1)
|
223 (50.9)
|
|
|
|
Hb, mean ± SD
|
11.6 ± 2.3
|
11.2 ± 2.0
|
12.1 ± 2.5
|
Male: 11.5–15.5 g/dL
Female: 11–14.5 g/dL
|
|
|
Platelets, mean ± SD
|
282.4 ± 144.1
|
304.2 ± 146.4
|
261.2 ± 139.0
|
140–400 109/L
|
0.001
|
|
Platelet volume, mean ± SD
|
8.1 ± 1.6
|
8.1 ± 1.7
|
8.2 ± 1.4
|
7.2–10.5 fL
|
0.580
|
|
HbA1c%, mean ± SD
|
6.8 ± 2.3
|
6.6 ± 2.1
|
6.9 ± 2.4
|
4–6%
|
0.423
|
|
HbA1c count, mean ± SD
|
50.5 ± 24.
|
48.4 ± 21.9
|
52.4 ± 26.5
|
≤ 42 mmol/mol
|
0.252
|
|
RBS, mean ± SD
|
|
7.1 ± 3.4
|
7.3 ± 3.5
|
3–5.6 mmol/L
|
0.674
|
|
LDL, mean ± SD
|
2.9 ± 1.3
|
3.0 ± 1.2
|
2.82 ± 1.30
|
< 2 mmol/L
|
0.226
|
|
PT, mean ± SD
|
14.5 ± 10.8
|
14.9 ± 14.0
|
14.0 ± 6.3
|
9.1–11.6 s
|
0.398
|
|
APTT, mean ± SD
|
40.2 ± 24.6
|
38.9 ± 25.5
|
41.5 ± 23.6
|
27.2–39.1 s
|
0.275
|
|
INR, mean ± SD
|
1.3 ± 0.9
|
1.4 ± 1.1
|
1.3 ± 0.7
|
2–4 ratio
|
0.462
|
|
Albumin, mean ± SD
|
28.8 ± 7.2
|
28.5 ± 7.2
|
29.1 ± 7.3
|
35–50 g/L
|
0.415
|
|
Protein C, n (%)
|
2 (0.5)
|
0 (0.0)
|
2 (0.5)
|
64–128 IU/dL
|
0.073
|
|
Protein S, n (%)
|
2 (0.5)
|
0 (0.0)
|
2 (0.5)
|
60–124 IU/dL
|
0.046
|
Hb: hemoglobin; HbA1c: glycated hemoglobin; RBS: random blood sugar; LDL: low-density lipoprotein; PT: prothrombin time; APTT: activated partial thromboplastin time; INR: international normalized ratio.
The mean glycated Hb was 6.8±2.3 and the mean low-density lipoprotein was 2.9±1.3. The mean albumin was 28.8±7.26 (females 28.5±7.2; males 29.1±7.3) as shown in Table 4.
Table 5 shows the various imaging modalities included in the study apart from the CTPA. Kidney-ureter-bladder ultrasound was found to be abnormal in 68 (15.5%) patients; nonspecific abnormal CXR was reported in 265 (60.5%). Nearly half of the patients 216 (49.3%) had abnormal echo, and more than a quarter each had diastolic dysfunction (125; 28.5%) and/or valve abnormalities (121; 27.6%).
Table 5: Radiological data of Omani patients with pulmonary embolism (N = 438).
|
Total patients
|
438 (100)
|
215 (49.1)
|
223 (50.9)
|
|
|
Abnormal ECG
|
189 (43.2)
|
94 (21.5)
|
95 (21.7)
|
0.477
|
|
Abnormal KUB US
|
68 (15.5)
|
26 (5.9)
|
42 (9.6)
|
0.256
|
|
Main CTPA
|
238 (54.3)
|
120 (27.4)
|
118 (26.9)
|
|
|
Segmental CTPA
|
343 (78.3)
|
164 (37.4)
|
179 (40.9)
|
|
|
Subsegmental CTPA
|
280 (63.9)
|
135 (30.8)
|
145 (33.1)
|
|
|
Abnormal echo
|
216 (49.3)
|
99 (22.6)
|
117 (26.7)
|
0.040
|
|
Ejection fraction
|
50.4 (13.4)
|
52.5 (11.9)
|
48.4 (14.5)
|
0.008
|
|
Valve abnormal
|
121 (27.6)
|
64 (14.6)
|
57 (13.0)
|
0.330
|
|
Diastolic dysfunction
|
125 (28.5)
|
55 (12.6)
|
70 (16.0)
|
0.121
|
|
Systolic dysfunction
|
54 (12.0)
|
23 (5.3)
|
31 (7.1)
|
0.263
|
ECG: electrocardiography; KUB US: kidney-ureter-bladder ultrasound; CTPA: computed tomography pulmonary angiogram; echo: echocardiography;
CXR: chest X-ray.
In the acute presentation of PE, 38 (8.7%) patients received reteplase and 399 (91.1%) patients received heparin. Warfarin was given to 281 (64.2%) patients and 134 (30.6%) patients received direct oral anticoagulants. Inferior vena cava (IVC) filter was inserted in 11 (2.5%) patients [Figure 2].
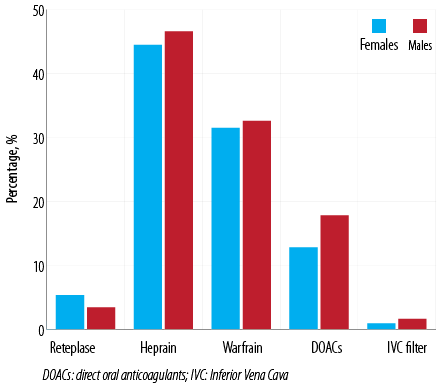 Figure 2: Drugs administered to Omani patients with pulmonary embolism (N = 438).
Figure 2: Drugs administered to Omani patients with pulmonary embolism (N = 438).
A total of 122 (27.9%) patients succumbed to death as shown in Figure 3. The causes of mortality were: PE (68; 15.5%), malignancy (18; 4.1%), septic shock (21; 4.8%), cardiogenic shock (5; 1.1%), and COVID-19 (5 1.1%). Among the 68 patients who died of PE, 16 had received reteplase. There was no significant difference between males and females regarding the death toll directly attributable to PE (36 vs. 32) [Table 6].
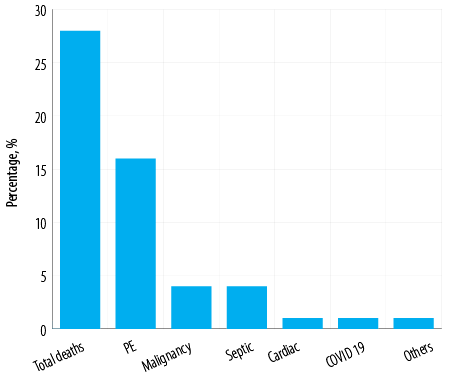 Figure 3: Numbers and causes of 122 (27.9%) deaths among Omani patients with pulmonary embolism (PE) (N = 438).
Figure 3: Numbers and causes of 122 (27.9%) deaths among Omani patients with pulmonary embolism (PE) (N = 438).
Discussion
Table 6: Death by group age among 438 of patients with acute pulmonary embolism.
|
< 40
|
13 (22.8)
|
13 (20.0)
|
52 (32.9)
|
51 (32.3)
|
65 (30.2)
|
64 (28.7)
|
129 (29.5)
|
|
40–65
|
27 (47.4)
|
22 (33.8)
|
65 (41.1)
|
65 (41.1)
|
92 (42.8)
|
87 (39.0)
|
179 (40.9)
|
|
> 65
|
17 (29.8)
|
30 (46.2)
|
41 (25.9)
|
42 (26.6)
|
58 (27.0)
|
72 (32.3)
|
130 (29.7)
|
|
Total
|
57
|
65
|
158
|
158
|
215
|
223
|
438
|
In the present study, the incidence of acute PE was more common among the relatively young and overweight participants. Our male patients had a higher mean age while females had a higher mean BMI. Recent surgery was the most common risk factor associated with PE. Almost two-thirds of our patients had HTN and one-third had DM with fewer patients reporting other risk factors such as malignancy, smoking, and other organ failures. The most common presentation symptom of PE was dyspnea and chest pain, and fewer patients presented with syncope and hemoptysis. PE was the most common direct cause of mortality among our patients compared to others, such as malignancy and shock.
Several studies reported that acute PE may affect males and females more or less equally.21,22 One study reported that PE-specific data regarding sex differences was limited and not well understood.22 However, there was a sex difference in the incidence of PE in older patients.23 Contrary to our findings, a study from the USA showed that women in the age group of 20–40 years had a higher risk of PE.24 However, a study from the Netherlands found that men aged 60–80 were at higher risk.25
The current study’s participants had high BMI (mean = 29.7), and females had higher BMI than males. A Saudi retrospective observational study found obesity to be the most common risk factor for PE.26 A German study also found high BMI in PE patients but did not report any significant difference in mean BMI between men and women.27
In this study, the most common risk factor for PE was recent surgery, which accounted for 24.9% of our PE patients. A Saudi study also reported a corresponding prevalence of 20%.26 A French study among cancer-free middle-aged PE patients found that the risk for PE was high during early (1–6 weeks) and late (6–12 weeks) postoperative in all types of surgeries.28
In our study, 16.7% of PE patients had an underlying malignancy. A study in the USA reported that malignancy increased the risk for PE by fourfold compared to the general population.29
The present study found that 11.2% of patients who presented with PE were bedbound. A retrospective Saudi study of 38 intensive care unit patients showed that immobilization (23.7%) was the most common risk factor for PE.30 A Spanish registry with 18 023 PE patients showed that immobilization doubled the risk for PE.31
Of our 215 female patients, 21 reported pregnancy at the time of presentation. A USA study reported that 9% of all pregnancy-related deaths were caused by PE, and that cesarean delivery raised the risk.32
Smoking is considered a risk factor for PE, especially in men.33 In our study, smoking habit was not a major risk factor. In addition, our female patients were far less likely to smoke than our male patients. A similar sharp difference between sexes was reported from Saudi Arabia as well.26 The unusually low prevalence of female smokers might be attributable to the cultural norms of the Arabian Gulf region.
HTN and DM were the most common diseases reported in our PE patients. A meta-analysis of 21 studies comprising 63 552 patients found HTN and DM to be important risk factors for venous thromboembolism.34
Among our patients, 10.5% had CKD which was significantly more common in males than in females. The association between CKD and PE can be explained by the pathophysiology of the procoagulant state in CKD patients as a result of an increase in von Willebrand factor, fibrinogen, VIIa and XIIa, and the reduction in plasminogen activator inhibitor-1.35 In addition, the use of erythropoietin agents in CKD patients increases their risk of thrombosis. A meta-analysis that involved 225 000 patients reported that renal impairment is a predictor of short-term and long-term adverse outcomes and mortality in patients with acute PE.36 One study showed that there was no sex difference in mortality in patients with CKD and PE.37
The most common presentation symptom for our patients was dyspnea, followed by chest pain and cough. All presentations had a similar frequency in both sexes except for hemoptysis, which was significantly more common in males. A Saudi study reported similar prevalence for PE presentations, the most common ones being dyspnea followed by chest pain.26 Other studies also showed that acute PE presentations usually present with dyspnea (56–89% of patients) followed by chest pain with insignificant male-female differences.33,38,39 An exception was syncope, accounting for 2–7% of PE patients, and is more common in females than males,40 which might be because females usually have more severe presentations. Similar to our study, hemoptysis has been found elsewhere to be more common in males.33
Several studies have shown that females are likely to have more severe presentations with hypotension, shock, high B-type natriuretic peptide, and abnormal echo findings such as RV dysfunction.22,38,39,41
Our patients with PE also had low Hb and lower-than-normal platelet counts. Males had lower mean platelet count while females had lower mean Hb level. Low Hb and low blood viscosity can lead to dysfunction in the vessel endothelium antithrombotic mechanism, which puts the patient at high risk for developing thrombosis.42
This study showed no significant difference between sexes in CTPA and ECG findings. Although CTPA is a very important imaging modality in diagnosing PE, only a few studies have investigated sex differences in CTPA findings in the diagnosis of PE. A trial in the USA—Prospective Investigation of Pulmonary Embolism Diagnosis II—showed that CTPA had higher specificity for females but similar sensitivity for both sexes (93% vs. 97%; p = 0.015) in diagnosing PE.43 However, a study from Germany reported that the patient’s sex had no effect on the pulmonary blood flow revealed by CTPA.44
More than half of the patients in this study had abnormal echo. The average ejection fraction was 50.4 (males = 48.4; females = 52.5). In addition, males had a tendency for systolic and diastolic dysfunctions while more females were likely to have valvular dysfunctions. A study from Germany that involved > 47 000 patients identified a significantly higher number of females who had RV dysfunction on echo.41 In addition, a 10-year prospective study reported that females were more likely to have evidence of RV dysfunction on echo.2
Studies suggest that patients with heart failure have a double risk of developing PE. In addition, a large cohort studies reported that decompensated heart failure was an independent risk factor of mortality in patients with PE.45 The increased incidence of PE in patients with heart failure can be related to the fact that reduced cardiac output can lead to reduced flow, abnormalities in platelet function, endothelial function, and hemostasis.45,46
In our study, most acute PE cases were managed with anticoagulation therapy. Heparin was the most used anticoagulant, followed by warfarin and direct oral anticoagulants. Only 38 patients received thrombolysis. Patients with absolute contraindications to systemic anticoagulation can be managed by inserting IVC filter.45,46 In the current study, IVC filter was the least utilized method of management, and it was used in males more than in females.
A German prospective observational trial suggested that females were more likely to be managed with thrombolysis (9.2% vs. 16.4%, p = 0.013).47 Nevertheless, a retrospective Japanese cohort study reported the opposite result that females were less likely than males to receive thrombolysis.22 On the other hand, a number of studies from Spain, the USA, and Japan did not find any sex-related differences in thrombolysis therapy.38,39 Another study found that females were less likely to receive IVC filters than males.39
In our study, 27.9% of patients died and 15.5% of deaths were directly attributed to PE. Though our female patients presented with more severe PE, mortality was higher in male patients. A Saudi retrospective observational study with 92 patients reported 15.2% mortality.48 Regarding the prevalence of PE related to patient sex, the data was mixed. A two-decade-old study based on the International Cooperative Pulmonary Embolism Registry reported higher male mortality, similar to our finding.33 However, some newer studies reported higher female mortality.38,39 Yet another study found no sex difference in mortality both in the short and long term.49 Such wide variation in mortality and morbidity data might be due to genetic, environmental factors, and possibly health-system-related factors.
This study has a few limitations, including that it was a retrospective design and the fact that it was a single-center study. We have also not processed age-related PE and mortality risks. We emphasize the need for a large prospective multicenter trial that studies more variables in Omani PE patients in short, medium, and long terms.
Conclusion
The present study showed that the incidence of acute PE was common in relatively young and overweight populations. Surgery was the most common risk factor for PE, followed by malignancy, smoking, and organ failures. Almost two-thirds of the patients who were presented with PE had HTN and one-third had DM. The most common presentation of PE was dyspnea and chest pain with fewer patients presenting with syncope and hemoptysis. Although most patients received anticoagulation therapy, PE was the most common direct cause of mortality.
This study has shown the great need for an individualized patient assessment for optimum management. Regular long-term follow-up of high-risk PE patients is essential.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
We are grateful to Dr. Fatima Sulaiyman Said Al Abri, a second-year radiology resident, and Mr. Dinesh Kumar, information technology administrator of picture archiving and communication system at the Royal Hospital, for their help.
references
- 1. Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al; ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol 2014 Nov;34(11):2363-2371.
- 2. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res 2016 Apr;118(9):1340-1347.
- 3. Crous-Bou M, Harrington LB, Kabrhel C. Environmental and genetic risk factors associated with venous thromboembolism. Semin Thromb Hemost 2016 Nov;42(8):808-820.
- 4. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al; The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J 2019 Oct;54(3):543-603.
- 5. Ji QY, Wang MF, Su CM, Yang QF, Feng LF, Zhao LY, et al. Clinical symptoms and related risk factors in pulmonary embolism patients and cluster analysis based on these symptoms. Sci Rep 2017 Nov;7(1):14887.
- 6. Abdel Hammed MR, Elgendy SG, El-Mokhtar MA, Sayed D, Mansour SM, Darwish AM. T-lymphocytes expression of toll-like receptors 2 and 4 in acute myeloid leukemia patients with invasive fungal infections. Mediterr J Hematol Infect Dis 2022 Mar;14(1):e2022022.
- 7. Lu Y, Dai J, Liu Q, Cai P, Zheng J. Screening of potential hub genes in pulmonary thromboembolism. Exp Ther Med 2022 Jan;23(1):18.
- 8. Mohammed D, Khallaf S, El-Naggar M, Abdel-Hameed M, Bakry R. Interleukin-10: a potential prognostic marker in patients with newly diagnosed multiple myeloma. Resum Oncol 2021;17(1):38-41.
- 9. Bontekoe E, Brailovsky Y, Hoppensteadt D, Bontekoe J, Siddiqui F, Newman J, et al. Upregulation of inflammatory cytokines in pulmonary embolism using biochip-array profiling. Clin Appl Thromb Hemost 2021;27:10760296211013107.
- 10. Abdel Hammed MR, Ahmed YA, Adam EN, Bakry R, Elnaggar MG. sVCAM-1, and TGFβ1 in chronic phase, chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Egypt J Immunol 2022 Oct;29(4):163-173.
- 11. Inami N, Nomura S, Kikuchi H, Kajiura T, Yamada K, Nakamori H, et al. P-selectin and platelet-derived microparticles associated with monocyte activation markers in patients with pulmonary embolism. Clin Appl Thromb Hemost 2003 Oct;9(4):309-316.
- 12. Abdel Hameed MR, Nafady HA, Mostafa MI, Sayed D, Obiedallah AA. Possible role of CD11a in primary immune thrombocytopenia patients on immunosuppressive therapy. J Blood Med 2021 Mar;12:197-205.
- 13. Tan Y, Yan M, Cheng Z, Pan X. Pulmonary thromboembolism in immune thrombocytopenia: a report of five cases and a review of the literature. Int J Gen Med 2021 Aug;14:4479-4483.
- 14. Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003 Feb;24(4):366-376.
- 15. Elliott CG, Goldhaber SZ, Visani L, DeRosa M. Chest radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry. Chest 2000 Jul;118(1):33-38.
- 16. Shopp JD, Stewart LK, Emmett TW, Kline JA. Findings from 12-lead electrocardiography that predict circulatory shock from pulmonary embolism: systematic review and meta-analysis. Acad Emerg Med 2015 Oct;22(10):1127-1137.
- 17. Febra C, Macedo A. Diagnostic role of mean-platelet volume in acute pulmonary embolism: a meta-analysis and systematic review. Clin Med Insights Circ Respir Pulm Med 2020 Oct;14:1179548420956365.
- 18. Abdel Hammed MR, El-Amien HA, Asham MN, Elgendy SG. Can platelets indices and blood neutrophil to lymphocyte ratio be used as predictors for diagnosis of spontaneous bacterial peritonitis in decompensated post hepatitis liver cirrhosis? Egypt J Immunol 2022 Oct;29(4):12-24.
- 19. Karamat A, Awan S, Hussain MG, Al Hameed F, Butt F, Wahla AS. Usefulness of clinical prediction rules, d-dimer, and arterial blood gas analysis to predict pulmonary embolism in cancer patients. Oman Med J 2017 Mar;32(2):148-153.
- 20. Alkindi S, Al-Ghadani AR, Al-Zeheimi SR, Alkindi SY, Fawaz N, Ballas SK, et al. Predicting risk factors for thromboembolic complications in patients with sickle cell anaemia - lessons learned for prophylaxis. J Int Med Res 2021 Dec;49(12):3000605211055385.
- 21. Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med 2013 Sep;126(9):832.e13-832.e21.
- 22. Yoshikawa Y, Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, et al; COMMAND VTE Registry Investigators. Sex differences in clinical characteristics and outcomes of patients with venous thromboembolism - from the COMMAND VTE registry. Circ J 2019 Jun;83(7):1581-1589.
- 23. Roach RE, Cannegieter SC, Lijfering WM. Differential risks in men and women for first and recurrent venous thrombosis: the role of genes and environment. J Thromb Haemost 2014 Oct;12(10):1593-1600.
- 24. Spencer FA, Emery C, Joffe SW, Pacifico L, Lessard D, Reed G, et al. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis 2009 Nov;28(4):401-409.
- 25. Engbers MJ, van Hylckama Vlieg A, Rosendaal FR. Venous thrombosis in the elderly: incidence, risk factors and risk groups. J Thromb Haemost 2010 Oct;8(10):2105-2112.
- 26. Al Dandan O, Hassan A, AbuAlola H, Alzaki A, Alwaheed A, Alalwan M, et al. Clinical and imaging profiles of pulmonary embolism: a single-institution experience. Int J Emerg Med 2020 Aug;13(1):47.
- 27. Keller K, Rappold L, Gerhold-Ay A, Hobohm L, Hasenfuß G, Konstantinides SV, et al. Sex-specific differences in pulmonary embolism. Thromb Res 2019 Jun;178:173-181.
- 28. Caron A, Depas N, Chazard E, Yelnik C, Jeanpierre E, Paris C, et al. Risk of pulmonary embolism more than 6 weeks after surgery among cancer-free middle-aged patients. JAMA Surg 2019 Dec;154(12):1126-1132.
- 29. Hisada Y, Geddings JE, Ay C, Mackman N. Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost 2015 Aug;13(8):1372-1382.
- 30. Harbi A, AlSamani AN, Adawi NM, Alswyan R, AlDhilan MM, Alajlan SA, et al. Thromboembolic diseases among intensive care unit patients in Al-Qassim Region, Saudi Arabia. Cureus 2022 Dec;14(12):e33033.
- 31. Nauffal D, Ballester M, Reyes RL, Jiménez D, Otero R, Quintavalla R, et al; RIETE Investigators. Influence of recent immobilization and recent surgery on mortality in patients with pulmonary embolism. J Thromb Haemost 2012 Sep;10(9):1752-1760.
- 32. Abe K, Kuklina EV, Hooper WC, Callaghan WM. Venous thromboembolism as a cause of severe maternal morbidity and mortality in the United States. Semin Perinatol 2019 Jun;43(4):200-204.
- 33. McHugh KB, Visani L, DeRosa M, Covezzoli A, Rossi E, Goldhaber SZ. Gender comparisons in pulmonary embolism (results from the International Cooperative Pulmonary Embolism Registry [ICOPER]). Am J Cardiol 2002 Mar;89(5):616-619.
- 34. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008 Jan;117(1):93-102.
- 35. Sagripanti A, Cupisti A, Baicchi U, Ferdeghini M, Morelli E, Barsotti G. Plasma parameters of the prothrombotic state in chronic uremia. Nephron 1993;63(3):273-278.
- 36. Xing X, Liu J, Deng Y, Xu S, Wei L, Yang M, et al. Impact of renal function on the prognosis of acute pulmonary embolism patients: a systematic review and meta-analysis. Expert Rev Respir Med 2022 Jan;16(1):91-98.
- 37. Panigada G, Masotti L, Rosi C, Teghini L, Cimolato B, Bertieri MC, et al; TUSCAN-PE Study Investigators (see end of article for the complete list of TUSCAN-PE Study Investigators). Thromboembolic burden, prognostic assessment and outcomes of females compared to males in acute pulmonary embolism. Acta Clin Belg 2016 Jun;71(3):142-148.
- 38. Barrios D, Morillo R, Guerassimova I, Barbero E, Escobar-Morreale H, Cohen AT, et al. Sex differences in the characteristics and short-term prognosis of patients presenting with acute symptomatic pulmonary embolism. PLoS One 2017 Nov;12(11):e0187648.
- 39. Tanabe Y, Yamamoto T, Murata T, Mabuchi K, Hara N, Mizuno A, et al. Gender differences among patients with acute pulmonary embolism. Am J Cardiol 2018 Sep;122(6):1079-1084.
- 40. Keller K, Hobohm L, Münzel T, Ostad MA, Espinola-Klein C. Syncope in haemodynamically stable and unstable patients with acute pulmonary embolism - Results of the German nationwide inpatient sample. Sci Rep 2018 Oct;8(1):15789.
- 41. Blanco-Molina A, Enea I, Gadelha T, Tufano A, Bura-Riviere A, Di Micco P, et al; RIETE Investigators. Sex differences in patients receiving anticoagulant therapy for venous thromboembolism. Medicine (Baltimore) 2014 Oct;93(17):309-317.
- 42. Irace C, Scarinci F, Scorcia V, Bruzzichessi D, Fiorentino R, Randazzo G, et al. Association among low whole blood viscosity, haematocrit, haemoglobin and diabetic retinopathy in subjects with type 2 diabetes. Br J Ophthalmol 2011 Jan;95(1):94-98.
- 43. Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al; PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006 Jun;354(22):2317-2327.
- 44. Meinel FG, Graef A, Sommer WH, Thierfelder KM, Reiser MF, Johnson TR. Influence of vascular enhancement, age and gender on pulmonary perfused blood volume quantified by dual-energy-CTPA. Eur J Radiol 2013 Sep;82(9):1565-1570.
- 45. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999 Apr;353(9162):1386-1389.
- 46. Beemath A, Stein PD, Skaf E, Al Sibae MR, Alesh I. Risk of venous thromboembolism in patients hospitalized with heart failure. Am J Cardiol 2006 Sep;98(6):793-795.
- 47. Keller K, Hobohm L, Ebner M, Kresoja KP, Münzel T, Konstantinides SV, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J 2020 Jan;41(4):522-529.
- 48. Khan NA, Alharbi AF, Alshehri AQ, Attieh AI, Farouk HH, Alshammri HH, et al. Early diagnosis of pulmonary embolism related to clinical presentation and vital signs in the emergency department at King Saud Medical City. Cureus 2022 Jul;14(7):e27087.
- 49. Marshall AL, Bartley AC, Ashrani AA, Pruthi RK, Durani U, Gonsalves WI, et al. Sex-based disparities in venous thromboembolism outcomes: A National Inpatient Sample (NIS)-based analysis. Vasc Med 2017 Apr;22(2):121-127.