H epatitis C virus (HCV) belongs to the Flaviviridae family a positive-sense RNA virus1 that leads toward hepatocellular carcinoma with 3% worldwide prevalence and is reported to be a vertically transmitted virus.2 In Pakistan, the health standards and living status of 170 million people are below average.1 According to an estimate, 10 million people in Pakistan are positive for HCV virus.3 The pathogenesis of HCV is based on biomarkers of oxidative stress (i.e., reactive oxygen species (ROS) and reactive nitrogen species). HCV proteins induce the production of free radicals in hepatocytes in the form of lipid peroxidation and oxidized thioredoxin.4 Moreover, excessive iron uptake causes iron deposits in the liver to react with ROS and produce more free radicals.5 The body regulates the biomarkers of oxidative stress with reductants and oxidative enzymes. During oxidative stress, levels of antioxidant enzymes get increased to counter the stress.5
Bacterial vaginosis (BV) is a lower reproductive tract abnormal condition. It causes considerable public health expenditures and emotional distress in females.6 Factors like hormonal fluctuation, use of contraceptives, douching, or promiscuous sexual practices disturb the healthy microflora followed by a growth of harmful aerobic or anaerobic bacteria leading to the formation of grayish vaginal discharge with fishy odor.7 Three out of the following four parameters are indicative of BV: vaginal pH ≥ 4.7, release of fishy odor after adding 10% potassium hydroxide, presence of ‘clue cells’ (stippled appearance of epithelial cells when covered with bacteria), and a thin, grey homogeneous discharge.8
In young females, Lactobacillus spp. colonizes the vaginal environment under the influence of estrogen which produces lactic acid to set the pH to 3.8–4.5.9 This acidic pH is particularly important for healthy microflora. The reduced number of Lactobacilli replaced by pathogenic anaerobic and aerobic flora produces aminopeptidases to generate amines, which increases the vaginal pH. Additionally, this abnormal flora produces enzymes and metabolic by-products that suppress the immune response and contribute to the severity of the infection and complications in pregnancy. Rupture of membranes, preterm delivery, or postpartum sepsis have been documented as associated issues with BV.10,11
Based on the demographic data of the study population, the prevalence of BV ranges from 4–64% while 12–25% of females have asymptomatic BV.12 Both BV and HCV infection lead to the development of oxidative stress which is the imbalance between generation and quenching of ROS.13,14 This leads to increased levels of free radicals including O2 and hydrogen peroxide (H2O2). ROS species have detrimental effects on the cellular integrity, also linked with apoptosis of affected cells. These cells release antioxidants to quench ROS species which include enzymes like catalase, peroxidase, and superoxide dismutase (SOD) to convert into water and oxygen. ROS species and their respective enzymes can serve as markers of oxidative stress.15 This concomitant presence of oxidative markers in HCV infection and BV may serve as a connection between the two conditions.
The disease severity is even higher when the patient has secondary infections. BV is reported to be linked with the colonization and transmission of sexually transmitted viruses including HIV, human papillomavirus, and herpes simplex virus.15 Although there are rare chances of venereal transmission of HCV, the strong relationship of having both BV and HCV led to the development of the study with the aim to compare the oxidative stress status in HCV-BV coinfection with respect to BV mono-infection among pregnant females (PFs).
Methods
A case-control study was designed enrolling PFs with HCV-BV coinfection and BV mono-infection compared with the healthy subjects (i.e., PF with no infection). All patients were selected from the Gynecology and Obstetrics outpatient department at Hilal-e-Ahmar Maternity Hospital, Punjab, Pakistan after taking the ethical approval (letter no. 539/HAHF/9-9-2016). Volunteer PFs (n = 200) were chosen for this study and consent forms were taken after verbal and written explanation of the methods and risk were given.
These females were divided into three groups; PF with HCV-BV (n = 25), PF with BV (n = 25), and PF control group (n = 25). Inclusion criteria for PF with HCV-BV was based on the following parameters: (a) pregnancy, (b) HCV detection, (c) per vaginal (PV) examination for BV.
Pregnancy was already determined by urine pregnancy strip test beta hCG (AccuMed)®. This test uses antibodies to detect hCG. It is an ideal marker of pregnancy since it rises rapidly and consistently in early pregnancy with an accuracy of over 99 %.16 The pregnancy of selected individuals was further confirmed by radiographical imaging using an ultrasound machine. Diagnosis of HCV by rapid diagnostic kit (ctK)® followed by confirmation with reverse transcription-polymerase chain reaction (RT-PCR). BV status was confirmed by PV examination and the Amsel criteria of BV17 was considered as the inclusion criteria for patients enrolled in the respective group.
During PV examination, vaginal swabs were collected for the microbiological study and enriched in trypticase soya broth (TSB; Oxoid UK)® for 24 hours at 37 °C. Vaginal discharge was collected with the help of cotton swab and dipped into 2 mL of phosphate-buffered saline (pH 7.3) (Oxoid UK)® in falcon tubes to study oxidative stress and stored at -80 °C. Females with a history of hysterectomy, AIDS, hypersensitivity to vitamin C, and recent chemotherapy were excluded from the study. Furthermore, for hematological analysis, oxidative stress analysis, and HCV detection, 6 mL of blood was collected from the median cephalic vein and equally divided into vacutainer tube (BD)® with and without anticoagulant.
The participants of the HCV-BV group were subjected to HCV detection with the aid of RT-PCR (Humacylcer). The extraction of RNA from plasma was conducted using a NucleoSpin® kit. HCV detection was carried out using a Qigong kit (Germany) based upon the amplification of single copy 5´ UTR RNA sequence and measuring of the amplification product, and concentration growth using RT-PCR and fluorescence-labeled probes. HCV presence was detected by FAM fluorophore. Amplification of the internal control was visualized by HEX fluorophore.
Complete blood count analysis was conducted with the aid of an Abacus 5-part (Hungry) hematology analyzer. Hemoglobin, granulocytes, monocytes, leukocytes, and platelets were measured by laser light scattering technology. Oxidative stress was determined in blood plasma of all the subjects enrolled in the study.
Streaking of vaginal swab was done on Blood, MacConkey, CLED, Nutrient, and MRS media (Oxoid UK®). All were incubated at 37 °C for 24 hours aerobically and anaerobically. Biochemical identification was conducted. Total bacterial count per mL was counted with the help of the Breed smear method.18
16S rRNA gene was amplified for two representative isolates, using universal primers. The amplicons were purified using the QIAquick PCR purification kit Qiagen (Germany). Sequencing for molecular identification of isolates was performed using sequencing services by 1st Base (Malaysia). Sequences were submitted to the GenBank database and accession numbers were obtained.
Plasma and vaginal secretions from both group participants along with the healthy control group were analyzed for the measurement of oxidative stress level that includes SOD, catalase, and lipid peroxidation, malondialdehyde (MDA), and H2O2.
SOD activity was assessed by a colorimetric assay kit ab65354 (Abcam, Cambridge, MA, USA). The reagent utilized in the kit is a water-soluble tetrazolium salt (WST-1), which produces a water-soluble formazan dye that can be detected at 450 nm upon the reduction of WST-1 by superoxide anions. WST-1 reduction is inhibited by SOD, which catalyzes the dismutation of the superoxide anion to produce H2O2 and O2. Therefore, SOD activity was calculated based on the percent inhibition of WST-1 reduction, which in turn reflected the percent inhibition of the superoxide anions.
Catalase activity was assessed by a colorimetric assay kit ab83464 (Abcam, Cambridge, MA, USA). The catalase present in the samples reacts with H2O2 to produce water and oxygen. The unconverted H2O2 reacts with a probe to produce a product that measured at OD 570 nm.
MDA activity was assessed by a colorimetric assay kit ab118970 (Abcam, Cambridge, MA, USA). The MDA in the samples reacts with thiobarbituric acid (TBA) to generate MDA-TBA adduct. The MDA-TBA adduct measured at OD 532 nm. H2O2 activity was assessed by a colorimetric assay kit ab102500 (Abcam, Cambridge, MA, USA). The H2O2 in the samples reacted with horse radish peroxidase at OD 570 nm.
For descriptive statistics of continuous variables, means and SDs were calculated, whereas categorical variables were expressed as proportions. The normality of variables was evaluated by the Kolmogorov–Smirnov test. Comparisons between groups were assessed using the Kruskal–Wallis test followed by a post hoc Dunn’s multiple comparison test due to the small data set. Statistical significance was defined as p < 0.05. The SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) and GraphPad Prism were used for all statistical analyses.
Results
One hundred and fifty participants gave consent for inclusion in the study, of which 75 met the inclusion criteria and were divided into three study groups with 25 participants in each group. The demographic characteristics of all groups are presented in Table 1. There was no significant difference (p > 0.050) in the average age among both coinfected and mono-infected groups. While the average weight was significantly higher among the coinfected group compared to the mono-infected group (p < 0.050). Moreover, the average ratio of children was 1.0 and 2.0 in the mono-infected and coinfected groups, respectively.
Table 1: Demographic parameters.
|
PF with HCV-BV
|
25
|
2.0 ± 1.5
|
65.1 ± 7.5
|
31.0 ± 3.1
|
|
PF with BV
|
25
|
1.0 ± 1.3
|
74.3 ± 5.9*
|
30.0 ± 3.4
|
PF: pregnant female ; HCV: hepatitis C virus; BV: bacterial vaginosis.*statistically significant.
Complete blood count parameters reveal that the average granulocytes percentage (neutrophils, basophils, eosinophils, and monocytes) were observed significantly higher among the coinfected individuals compared to the mono-infected (p < 0.050). The average lymphocytes, platelets, and blood hemoglobin level were significantly lower among the coinfected individuals compared to the mono-infected individuals (p < 0.050). The results indicate the severity of disease among the coinfected group compared to the mono-infected group [Figure 1].
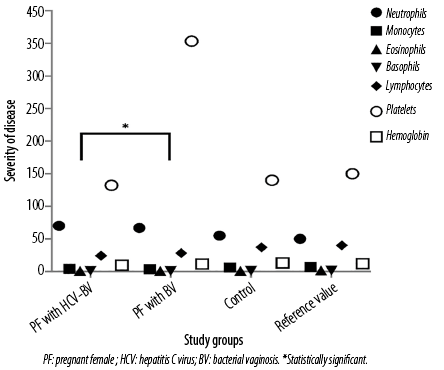 Figure 1: Hematological analysis.
Figure 1: Hematological analysis.
Overall, the coinfected group’s microbiological culture reports showed a significantly higher frequency of pathogenic isolate (i.e., Acinetobacter spp.). We observed that the Nugent score was comparatively high for the coinfected group [Table 2]. These results suggest the severity of disease in the coinfected group.
Table 2: Microbiological analysis and Nugent score.
|
PF with HCV-BV
|
-
|
-
|
100
|
2
|
19*
|
|
PF with BV
|
-
|
40
|
60
|
8
|
13
|
PF: pregnant female ; HCV: hepatitis C virus; BV: bacterial vaginosis. One representative isolate was sequenced for 16s rRNA and accession numbers were obtained from the National Center for Biotechnology Information. *statistically significant.
Percentage inhibition of SOD by the reducing superoxide anion was significantly higher in mono-infection compared to coinfection group (p < 0.050). The overall trends of superoxide anion reduction were more clearly observed in vaginal secretions than the blood plasma in all three study groups. A similar trend was observed for catalase produced [Figure 2, a and b]. These results suggested the decreasing trend of antioxidant levels among the coinfected group.
The MDA and H2O2 levels were significantly higher in the coinfected group compared to the mono-infected in blood plasma as well as vaginal secretions of both groups (p < 0.050). The results indicate greater oxidative stress in the case of coinfection [Figure 2, c and d].
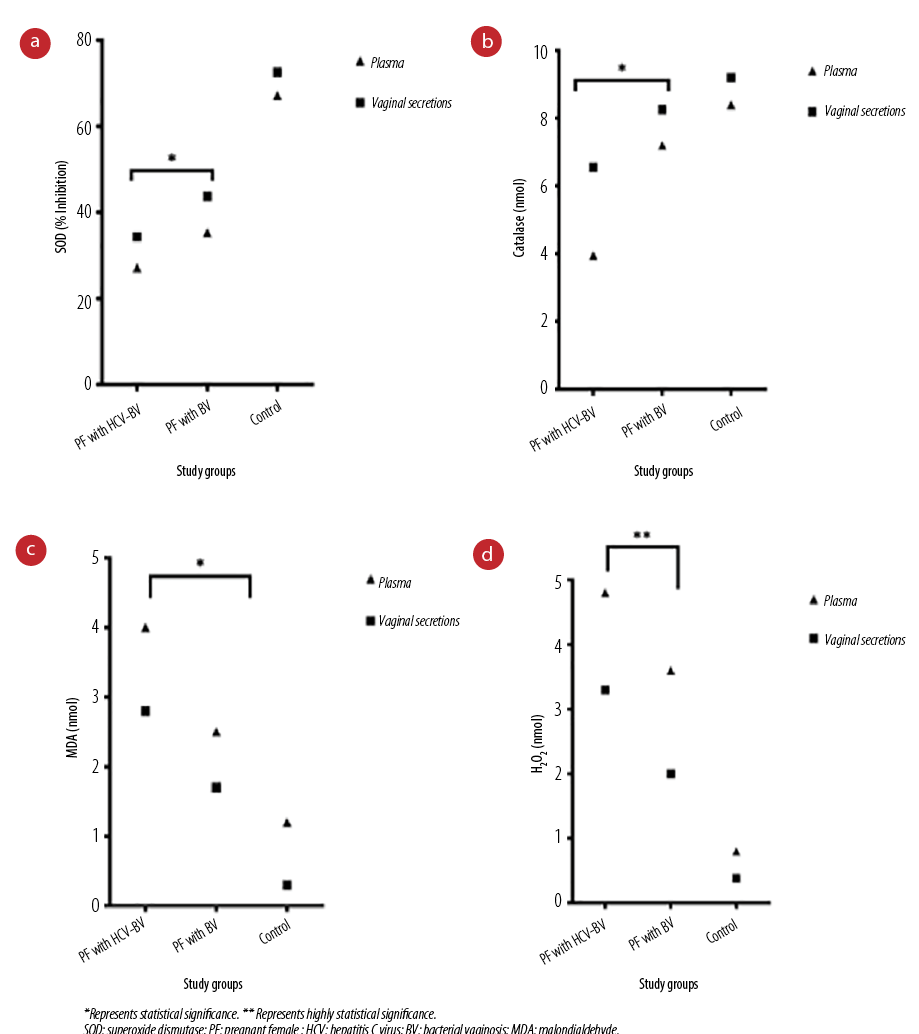 Figure 2: (a) Plasma and vaginal secretions SOD activity with a colorimetric assay kit. (b) Plasma and vaginal secretions catalase levels using the catalase assay kit. (c) Plasma and vaginal secretions MDA levels using the MDA assay kit. (d) Plasma and vaginal secretions H2O2 levels using the H2O2 assay kit.
Figure 2: (a) Plasma and vaginal secretions SOD activity with a colorimetric assay kit. (b) Plasma and vaginal secretions catalase levels using the catalase assay kit. (c) Plasma and vaginal secretions MDA levels using the MDA assay kit. (d) Plasma and vaginal secretions H2O2 levels using the H2O2 assay kit.
Discussion
In recent years, much attention has been given to the investigation of clinical biomarkers indicating the severity of infection in pregnancy and enabling early diagnosis and therapy. PFs are more severely affected by bacterial/viral infections and experience immunologic alteration compared to non-PFs.19 BV is the most common cause of abnormal vaginal discharge with a prevalence as high as 50% in non-pregnant communities and 9–23% in pregnant women.20 The main cause of BV is the disturbance in healthy microbiota, an important barrier against the pathogenic organism. The replacement of predominant lactobacillus spp. in the vagina replaced by different anaerobes is the most significant observation in the case of vaginosis.21 This study was designed to present the redox status as an important parameter for assessing the severity of the disease in HCV-BV coinfection, so that physicians can opt for better treatments and control the infection.
In this study, Acinetobacter spp. significantly out-numbered the Lactobacillus spp. (p < 0.050) among the coinfected group compared to the mono-infected and control groups [Table 1]. A similar study from Nepal suggested that BV has a direct association with Acinetobacter spp.22 Acinetobacter a well-known, commensal bacteria is multidrug resistant and the pathogen responsible for pneumonia, fever septicemia, chorioamnionitis, and premature contraction during pregnancy.23,24
Globally, BV is a common genital problem among women seeking gynecological care. In the current study, the average age of the affected participants was 31 years with no significant difference (p > 0.050) among both coinfected and mono-infected PF [Figure 1]. These findings are in accordance with the study from Nepal that reported a high prevalence of BV among the 31–40 age group.22 Similarly, in India, the prevalence rate of BV was 24.4% using Nugent’s method and this rate was higher among the 30–40 age group.25,26
A range of factors may explain this age and BV puzzle, such as late marriages, low fertility, and the demographic and socioeconomic status of the participants. The study suggests a more detailed demographic review conducted to gain insights in the factors that contribute the most. According to Pakistan’s Demographic and Health Survey, the average marriage age in Pakistan is 18.4 years. Age factor is the major determinant of fertility in females.27 In this study, the average ratio of the children among HCV-BV coinfected and BV-mono-infected was 1:2. Late marriages are another responsible factor where unmarried females may find greater opportunities to excel in their careers, but fertility issues increase with age. At the same time, late marriages concept was not acceptable in a society like Pakistan.28 Use of contraceptives and douching practices are also known causes of BV. Studies have been reported where douching was statistically related to BV (p = 0.015).29,30,31,32 All these factors are associated with altering the normal vaginal pH, and hence increase the risk factor for the acquisition of sexually transmitted infections including HIV and HCV.
Another interesting fact, HCV-BV significantly correlated (p < 0.050) with obesity compared to BV-mono-infected and higher Nugent score [Tables 1 and 2]. Similar trends of obesity and higher Nugent scores have been observed in studies reporting the association of obesity with HCV and BV.33,34 Weight gain during pregnancy is an obvious process, apart from physiological stress and diet, hormonal imbalance such as an increase in hypothalamic-pituitary-adrenal axis activity is directly associated with weight gain during pregnancy. However, a study from Washington, USA, suggested that there is no relationship between obesity and BV.35 This contradiction in the findings might be because of the ethnic and geographical distribution.
Globally, 71 million people are living with HCV out of which 7.1 million (10%) are present in Pakistan with the second largest HCV burden in the world.1,36 HCV causes chronic infection of the liver characterized by persistent inflammation leading to regenerative liver fibrosis and cirrhosis.37
Muriel P state that the immune response to chronic HCV infection not only initiates the production of oxidative stress but also leads to a higher level of oxidative stress when compared to other hepatitis viruses.4 Even its core proteins NS3, NS5A, E1, E2, and NS4B are directly involved in increase of oxidative stress.38,39
The presence of excess ROS can lead to cellular damage of DNA, lipids, and cellular protein.40 Similarly in the BV patients, the disturbance of healthy vaginal flora causes bulk production of ROS in the genital tract and is capable to cause vaginal epithelial cell apoptosis.13 Some other studies have also reported gynecological diseases including fibroids, endometriosis, and postoperative adhesions associated with oxidative stress.41–43
Noticeably, PFs experience more ROS production.44 During pregnancy physiological, anatomical, and metabolic changes occur therefore, the balance in ROS is very much crucial for the developing cells of the fetus and maturation. These ROS come from the mother's body during supply of adequate nutrients and oxygen.45
The sum of the above discussion reveals that BV, HCV, and pregnancy are directly involved in the oxidative stress in terms of ROS production. Therefore, we collectively study the oxidative stress in HCV-BV-coinfected PFs. Antioxidants are produced as a part of the physiological process of the cell to combat the ROS. They play a critical role in disease prevention by scavenging and detoxification of ROS and maintaining health. A decrease in the antioxidant defense system or overproduction of free radicals results in oxidative stress which induces aging. In this study, we found lower antioxidant levels in vaginal secretions among the coinfected, then mono-infected group [Figure 2a and b] indicating the severity of disease in coinfection. These results can be related to a study where bacterial/viral coinfection (HIV-TB) had high levels of antioxidants compared to mono-infection.46 However, in our study, the reason for lower levels of the antioxidant in vaginal secretion among the coinfected group is related to the ROS production. Further, we found high ROS production in blood plasma among the coinfected compared to the mono-infected group [Figure 2c and d]. These results are in accordance with a study where blood plasma ROS was significantly higher (p < 0.050), in the HIV-HCV coinfected participants compared to HIV mono-infected participants47 although this comparative study was based solely on viral diseases. A suggested reason for high ROS production in plasma is the HCV virus. However, further prospective studies are required to confirm this physiological condition.
Therapies based on both antioxidants and antioxidant enzymes can be an effective approach to preventing or treating diseases. Our study suggests the need for the molecular analysis of oxidative stress genes and computational approaches to find out the genetic bases and association of the disease which may provide insight knowledge to determine the exact reason.
This study is limited by the small number of participants and demographic factors, such as knowledge about douching and contraceptive use, diet, and living status. However, the study may provide baseline knowledge about the pathophysiology of coinfection. Moreover, the study lacks information about the therapeutic methods for HCV and BV patients, which may influence the results. Further study is required to analyze the impact of treatment among both study groups.
Conclusion
Overall, we found the severity of disease among the coinfected group. The patients from this group experienced more oxidative stress and pathogenic microbial burden. The levels of ROS and antioxidants can be used as diagnostic markers to label the disease severity, also antioxidants can be used as optimal therapeutic agents. This research could aid in the early diagnosis of hepatocellular cancer and BV, as well as encourage scientists to develop an oxidative stress marker for coinfection detection.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study. The 16s rRNA sequence of representative isolates was submitted under accession numbers MT269939 and MZ314512 on NCBI.
Acknowledgments
We acknowledge the role of Pathology Department of Faisalabad institute of Cardiology and Alkhidmat Diagnostic Center for providing lab facilities.
references
- 1. Waheed Y, Shafi T, Safi SZ, Qadri I. Hepatitis C virus in Pakistan: a systematic review of prevalence, genotypes and risk factors. World J Gastroenterol 2009 Dec;15(45):5647-5653.
- 2. Nesrine F, Saleh H. Hepatitis C virus (HCV) status in newborns born to HCV positive women performing intracytoplasmic sperm injection. Afr Health Sci 2012 Mar;12(1):58-62.
- 3. Hamid S, Umar M, Alam A, Siddiqui A, Qureshi H, Butt J; Pakistan Society of Gastroenterology. PSG consensus statement on management of hepatitis C virus infection–2003. J Pak Med Assoc 2004 Mar;54(3):146-150.
- 4. Muriel P. Role of free radicals in liver diseases. Hepatol Int 2009 Dec;3(4):526-536.
- 5. Pelusi S, Valenti L, Fargion S. Oxidative stress and hepatic iron overload, in studies on hepatic disorders. Springer; 2015. p. 345-356.
- 6. Beghini J, Giraldo PC, Riboldi R, Amaral RL, Eleutério J Jr, Witkin SS, et al. Altered CD16 expression on vaginal neutrophils from women with vaginitis. Eur J Obstet Gynecol Reprod Biol 2013 Mar;167(1):96-99.
- 7. Muzny CA, Schwebke JR. Pathogenesis of bacterial vaginosis: discussion of current hypotheses. J Infect Dis 2016 Aug;214(Suppl 1):S1-S5.
- 8. Petersen EE, Magnani P. Efficacy and safety of vitamin C vaginal tablets in the treatment of non-specific vaginitis. A randomised, double blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol 2004 Nov;117(1):70-75.
- 9. Amabebe E, Anumba DO. The vaginal microenvironment: the physiologic role of lactobacilli. Front Med (Lausanne) 2018 Jun;5:181.
- 10. Jacobsson B, Pernevi P, Chidekel L, Jörgen Platz-Christensen J. Bacterial vaginosis in early pregnancy may predispose for preterm birth and postpartum endometritis. Acta Obstet Gynecol Scand 2002 Nov;81(11):1006-1010.
- 11. Holst E, Goffeng AR, Andersch B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol 1994 Jan;32(1):176-186.
- 12. Piek CJ. Canine idiopathic immune-mediated haemolytic anaemia: a review with recommendations for future research. Vet Q 2011 Sep;31(3):129-141.
- 13. Chen Z, Zhang Z, Zhang H, Xie B. Analysis of the oxidative stress status in nonspecific vaginitis and its role in vaginal epithelial cells apoptosis. Biomed Res Int 2015 Oct 19;2015:795656.
- 14. Maki A, Kono H, Gupta M, Asakawa M, Suzuki T, Matsuda M, et al. Predictive power of biomarkers of oxidative stress and inflammation in patients with hepatitis C virus-associated hepatocellular carcinoma. Ann Surg Oncol 2007 Mar;14(3):1182-1190.
- 15. Lozano-Sepulveda SA, Bryan-Marrugo OL, Cordova-Fletes C, Gutierrez-Ruiz MC, Rivas-Estilla AM. Oxidative stress modulation in hepatitis C virus infected cells. World J Hepatol 2015 Dec;7(29):2880-2889.
- 16. Adkins S, Burmeister M. Visualization of DNA in agarose gels as migrating colored bands: applications for preparative gels and educational demonstrations. Anal Biochem 1996 Aug;240(1):17-23.
- 17. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983 Jan;74(1):14-22.
- 18. Wang S-H. A direct smear method for counting microscopic particles in fluid suspension. J Bacteriol 1941 Sep;42(3):297-319.
- 19. Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis 2006 Nov;12(11):1638-1643.
- 20. Nardis C, Mosca L, Mastromarino P. Vaginal microbiota and viral sexually transmitted diseases. Ann Ig 2013;25(5):443-456.
- 21. Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet 2014 Mar;289(3):479-489.
- 22. Ranjit E, Raghubanshi BR, Maskey S, Parajuli P. Prevalence of bacterial vaginosis and its association with risk factors among nonpregnant women: a hospital based study. Int J Microbiol 2018 Mar 5;2018:8349601.
- 23. Roca I, Espinal P, Vila-Farrés X, Vila J. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front Microbiol 2012 Apr;3:148.
- 24. Aivazova V, Kainer F, Friese K, Mylonas I. Acinetobacter baumannii infection during pregnancy and puerperium. Arch Gynecol Obstet 2010 Jan;281(1):171-174.
- 25. Modak T, Arora P, Agnes C, Ray R, Goswami S, Ghosh P, et al. Diagnosis of bacterial vaginosis in cases of abnormal vaginal discharge: comparison of clinical and microbiological criteria. J Infect Dev Ctries 2011 May;5(5):353-360.
- 26. Gad GF, El-Adawy AR, Mohammed MS, Ahmed AF, Mohamed HA. Evaluation of different diagnostic methods of bacterial vaginosis. IOSR Journal of Dental and Medical Sciences 2014;13(1):15-23.
- 27. National Institute of Population Studies Islamabad, Pakistan. Pakistan demographic and health survey 2017-2018. 2019 [cited 2023 March 27]. Available from: https://dhsprogram.com/pubs/pdf/FR354/FR354.pdf.
- 28. Hamid S, Stephenson R, Rubenson B. Marriage decision making, spousal communication, and reproductive health among married youth in Pakistan. Glob Health Action 2011 Jan;4(1):5079.
- 29. Ness RB, Hillier SL, Richter HE, Soper DE, Stamm C, McGregor J, et al. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet Gynecol 2002 Oct;100(4):765-772.
- 30. Hutchinson KB, Kip KE, Ness RB; Gynecologic Infection Follow-Through (GIFT) Investigators. Vaginal douching and development of bacterial vaginosis among women with normal and abnormal vaginal microflora. Sex Transm Dis 2007 Sep;34(9):671-675.
- 31. Brumley J. Testing a model of bacterial vaginosis among black women. Digital Commons. University of Digital Commons; 2012.
- 32. Vodstrcil LA, Hocking JS, Law M, Walker S, Tabrizi SN, Fairley CK, et al. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: a systematic review and meta-analysis. PLoS One 2013 Sep;8(9):e73055.
- 33. Tsao Y-C, Chen JY, Yeh WC, Peng YS, Li WC. Association between visceral obesity and hepatitis C infection stratified by gender: a cross-sectional study in Taiwan. BMJ Open 2017 Nov;7(11):e017117.
- 34. Brookheart RT, Lewis WG, Peipert JF, Lewis AL, Allsworth JE. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol 2019 May 1;220(5):476-e1-476-e11.
- 35. Lokken EM, Richardson BA, Kinuthia J, Mwinyikai K, Abdalla A, Jaoko W, et al. A prospective cohort study of the association between body mass index and incident bacterial vaginosis. Sex Transm Dis 2019 Jan;46(1):31-36.
- 36. Pradat P, Virlogeux V, Trépo E. Epidemiology and elimination of HCV-related liver disease. Viruses 2018 Oct;10(10):545.
- 37. Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol 2016 May;13(3):267-276.
- 38. Koike K. Hepatitis C virus contributes to hepatocarcinogenesis by modulating metabolic and intracellular signaling pathways. J Gastroenterol Hepatol 2007 Jun;22(Suppl 1):S108-S111.
- 39. Farshadpour F, Taherkhani R. Prevalence and molecular evaluation of hepatitis C virus infection among multi-transfused thalassemia patients in south of Iran. Oman Med J 2022 Sep;37(5):e427.
- 40. Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev 2016 Oct;2016:3164734.
- 41. Vural M, Camuzcuoglu H, Toy H, Camuzcuoglu A, Aksoy N. Oxidative stress and prolidase activity in women with uterine fibroids. J Obstet Gynaecol 2012 Jan;32(1):68-72.
- 42. Santulli P, Borghese B, Lemaréchal H, Leconte M, Millischer AE, Batteux F, et al. Increased serum oxidative stress markers in women with uterine leiomyoma. PLoS One 2013 Aug;8(8):e72069.
- 43. Fletcher NM, Saed MG, Abu-Soud HM, Al-Hendy A, Diamond MP, Saed GM. Uterine fibroids are characterized by an impaired antioxidant cellular system: potential role of hypoxia in the pathophysiology of uterine fibroids. J Assist Reprod Genet 2013 Jul;30(7):969-974.
- 44. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 2012 Jun;10(1):49.
- 45. Toboła-Wróbel K, Pietryga M, Dydowicz P, Napierała M, Brązert J, Florek E. Association of oxidative stress on pregnancy. Oxid Med Cell Longev 2020 Sep 15;2020:6398520.
- 46. Gravier-Hernández R, Gil-Del Valle L, Valdes-Alonso L, Hernández-Ayala N, Bermúdez-Alfonso Y, Hernández-Requejo D, et al. Oxidative stress in hepatitis C virus-human immunodeficiency virus co-infected patients. Ann Hepatol 2020;19(1):92-98.
- 47. Baum MK, Sales S, Jayaweera DT, Lai S, Bradwin G, Rafie C, et al. Coinfection with hepatitis C virus, oxidative stress and antioxidant status in HIV-positive drug users in Miami. HIV Med 2011 Feb;12(2):78-86.