Although pediatric adenoviral infection is usually mild and self-limiting, it can be severe in children with impaired cellular immunity, for whom disseminated forms are almost always fatal.1 However, only very few cases with acute myeloid leukemia (AML) have been reported to have fatal disseminated adenoviral disease.2
Case Report
A 15-month-old girl with AML (AML M0-M1) was being managed in our hospital as per AML Sheffield protocol. One week after chemotherapy course II, she developed worsening diarrhea and cough. Adenovirus was detected by polymerase chain reaction in stool and plasma level of 13 200 (log 4.1) copies/mL. She was managed conservatively, and the viral load was monitored weekly. Two weeks later, she became sicker with bilious vomiting, tachycardia, and progressive abdominal distension and tenderness. Intestinal perforation was ruled out by abdominal X-ray [Figure 1]. Computed tomography scan of the abdomen revealed diffusely distended, fluid-filled small and large bowels with increased enhancement. There was no focal mural thickening, hypo-enhancement, or pneumatosis intestinalis [Figure 2]. She was admitted to the pediatric intensive care unit (PICU) with gram-negative bacteremia complicated with septic shock that required inotropic support. She was started empirically on meropenem and vancomycin. Her blood culture showed Escherichia coli with sensitivity to amoxicillin/clavulanate, cefuroxime and gentamicin. Further blood cultures were negative with no growth. While in the PICU, she developed bloody diarrhea with significant blood loss. Laboratory evaluation revealed deranged coagulation, thrombocytopenia, and hemoglobin level dropped from 9 g/dL to 6 g/dL, requiring multiple blood transfusions, fresh frozen plasma, platelet transfusions, and intravenous vitamin K. Her plasma adenoviral titer increased to 17 200 copies/mL (log 4.2), so we started cidofovir (5 mg/kg weekly) and probenecid. Despite two doses of cidofovir, adenoviral load continued to increase to 1 130 000 copies/mL (log 6.1) and then to > 10 000 000 copies/mL (> log 7). Her stools and respiratory secretions were positive for enteric adenovirus, denoting disseminated adenoviral infection.
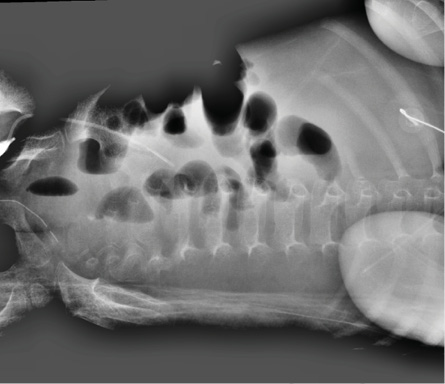 Figure 1: Left lateral decubitus abdominal X-ray. A few gaseous distended bowel loops are noted with a few scattered fluid levels, denoting stagnation. No evidence of pneumatosis or pneumoperitoneum is seen.
Figure 1: Left lateral decubitus abdominal X-ray. A few gaseous distended bowel loops are noted with a few scattered fluid levels, denoting stagnation. No evidence of pneumatosis or pneumoperitoneum is seen.
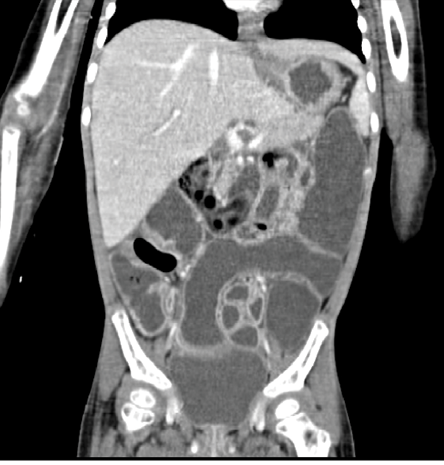 Figure 2: CT image of abdomen and pelvis intravenously and orally given contrasts showing hepatomegaly, diffuse bowel distension, and fluid-filled small and large bowel loops with increased enhancement.
Figure 2: CT image of abdomen and pelvis intravenously and orally given contrasts showing hepatomegaly, diffuse bowel distension, and fluid-filled small and large bowel loops with increased enhancement.
During her PICU stay, the patient remained severely neutropenic despite treatment with filgrastim. She had significant upper and lower gastrointestinal bleeding with sloughing of intestinal mucosa. Examination of the shed tissue revealed abundant blood clots mixed with occasional non-viable tissue with vague outlines resembling superficial mucosal surface. Bleeding diathesis was also presented with ecchymosis, gum bleeding, conjunctival bleeding, and microscopic hematuria. Supportive measures using multiple fresh frozen plasma, platelet transfusions, and activated factor VII failed to improve her bleeding diathesis. Ultimately, she had multisystem organ failure with refractory hypotension, renal shutdown, coagulopathy, and transaminitis. Her condition deteriorated further despite optimal supportive measures such as ventilator support, renal replacement therapy, broad-spectrum antimicrobial, and cidofovir therapy. The patient passed away due to uncontrolled disseminated adenoviral infection complicated with multi-organ dysfunction.
Discussion
Adenovirus infections are common among children, with 80% of 1–5-year-olds developing antibodies to one or more of its serotypes. Infections in immunocompetent hosts are usually benign and self-limiting, and manifest as mild respiratory, gastrointestinal, and/or ocular disease. In immunocompromised patients, especially transplant recipients, adenoviruses may cause severe infections, leading to morbidity and mortality.1 The risk and severity of adenoviral disease rise with increasing immunosuppression of the host. A Canadian report suggested that severe adenoviral infection in children with AML was rare and found only one such case, which had a fatal outcome.2
When our patient became seriously infected by disseminated adenovirus, she had already been administered two courses of intensive myelosuppressive chemotherapy (uncommon in this population) to combat her AML. It is possible that intensive chemotherapy made severe viral infection possible. In addition, it is known that AML interacts with the immune system in two distinct ways: immune editing and immune evasion. Immune editing is mediated by the production of soluble molecules that directly suppress the host lymphocytes, facilitating the induction of Treg. The AML cells also have multiple strategies to evade the host T cells and natural killer cells. These interacting mechanisms result in T-cell exhaustion, anergy and apoptosis, and natural killer cell suppression.3 Disseminated adenoviral infections reportedly have an overall fatality rate of 73% (83% among the immunodeficient and 60% in the immunocompetent).4 In immunocompromised patients, the ability of adenovirus to build up sufficient viral particles in the bloodstream leads to endothelial cell injury and progression to disseminated intravascular coagulopathy—further increasing the fatality risk.4
Early treatment might confer a better outcome. Cidofovir/probenecid combination has been successful in treating adenovirus infection in immunocompromised patients after hematopoietic stem cell transplantation (HSCT). The role of probenecid is to enhance the plasma concentration of the antiviral drug and to prevent renal toxicity.5 European Conference on Infections in Leukemia recommended deferring chemotherapy/conditioning whenever possible to prevent adenoviral dissemination.6 It is also recommended to check the adenoviral load in plasma in the affected children to guide early treatment.6 Once the viral load is > 1000 copies/mL, cidofovir therapy could start at 5 mg/kg/dose or 1 mg/kg/dose thrice a week. Among 70 HSCT recipients with disseminated adenovirus infection who received at least two doses of cidofovir, the mortality rate was reported to be 19%.7
Response to treatment and survival depends mainly on immune reconstitution/withdrawal of immunosuppression and early treatment. Our patient received two doses of cidofovir to combat her adenoviral infection. Although the first plasma adenovirus polymerase chain reaction titer was high (13 200 copies/mL (log 4.1)), treatment with cidofovir started only upon getting a rising titer the following week.
In a large cohort of pediatric recipients of HSCT, Bordigoni et al,8 reported successful treatment of adenoviral infection in 8 out of 22 patients. Ribavirin had a successful outcome in 3/13 patients, cidofovir in 2/3 patients, while ribavirin-cidofovir combination succeeded in one patient, and ribavirin with donor lymphocyte infusion in two patients.8
Conclusion
Disseminated adenoviral infection and hemorrhagic enterocolitis can be fatal in immunocompromised children with AML. Careful monitoring of clinical status and viral load with early initiation of antiviral therapy and appropriate supportive measures might be lifesaving.
Disclosure
The authors declared no conflicts of interest. The patient’s guardian gave his consent for the publication of this case.
references
- 1. Wy Ip W, Qasim W. Management of adenovirus in children after allogeneic hematopoietic stem cell transplantation. Adv Hematol 2013;2013:176418.
- 2. Renzi S, Ali S, Portwine C, Mitchell D, Dix D, Lewis V, et al. Adenovirus infection in children with acute myeloid leukemia: a report from the Canadian infection in acute myeloid leukemia research group. Pediatr Infect Dis J 2018 Feb;37(2):135-137.
- 3. Barrett AJ. Acute myeloid leukaemia and the immune system: implications for immunotherapy. Br J Haematol 2020;188(1):147-158.
- 4. Hussain SA, Zafar A, Faisal H, Vasylyeva O, Imran F. Adenovirus-associated disseminated intravascular coagulation. Cureus 2021 Mar;13(3):e14194.
- 5. Lindemans CA, Leen AM, Boelens JJ. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood 2010 Dec;116(25):5476-5485.
- 6. Ein HE, Eng DE, Hir HHH, Rob CR. Recommendation update 2019. 2019 [cited 2022 July 17]. Available from: https://www.ebmt.org/sites/default/files/2019-12/ECIL%208-Update%20on%20Community-acquired%20respiratory%20viruses%20in%20hematology%20patients%20-%20Final%20Slide%20Set.pdf.
- 7. Neofytos D, Ojha A, Mookerjee B, Wagner J, Filicko J, Ferber A, et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant 2007 Jan;(1):74-81.
- 8. Bordigoni P, Carret A-S, Ronique Venard V, Witz F, Le Faou A. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clinical Infectious Diseases 2001;32(9):1290-1297.