Tracheoesophageal fistula (TEF) is a surgically correctable congenital anomaly that manifests within the first few days or hours of life. TEF results from defects in the mesenchyme separating trachea and esophagus. Clinical presentation in neonates include respiratory distress, difficulty feeding, and excessive salivation, and the child may have coexisting anomalies like cardiovascular defects, renal agenesis, microcephaly, and duodenal atresia, etc.1–3 Its incidence is around 1 in every 3000 to 4500 births.4–6 TEF is classically repaired with surgical ligation and primary end-to-end anastomosis via right-sided thoracotomy7 under general anesthesia (GA) with or without regional anesthesia (RA). Preoperative anesthetic considerations in the management of TEF include anatomical variations in the airway, difficulty in endotracheal tube placement, dehydration, prematurity, neonatal respiratory distress syndrome, and ventilation of the lung in the presence of a fistula. Risk factors like prematurity, low birth weight, difficult airway, respiratory distress due to repeated aspiration, congenital heart diseases, and large defects contribute to the mortality.8
Analgesia in TEF repair surgery is usually achieved with pharmacological agents (opioids and NSAIDs) and RA techniques.9 Intravenous (IV) opioids have been used to maintain analgesia during both the intra-operative and postoperative period with fentanyl being one of the most commonly used opioid analgesic in neonates.10 It helps to maintain stable hemodynamics while avoiding stress responses. The increase in plasma fentanyl levels has been associated with significantly prolonged ventilatory support,11 and delayed extubation as neonates have a high volume of distribution, lower plasma clearance, decreased renal clearance of opioids, and decreased protein binding.
IV paracetamol can be used for treating moderate pain and when integrated in multimodal analgesia, had shown a clinically relevant reduction in opioid consumption.12 Intercostal nerve block (ICNB) can be used to provide analgesia in thoracotomy.13,14 It provides analgesia similar to epidural and systemic drugs while avoiding complications associated with epidural technique. ICNB is easy to perform, does not require much expertise, preserves somatic and diaphragmatic function, and provides localized analgesia. Enhanced recovery after surgery (ERAS) protocols advocate avoiding opioids in the perioperative period and providing multimodal analgesia including RA.15,16 This prompted us to explore methods to minimize the intraoperative anesthetic exposure and opioids to aid early extubation which accelerates recovery, shorten the length of hospital stay, and decrease the medical expense. Hence, we decided to compare extubation time and postoperative analgesia in neonates with TEF undergoing surgery using fentanyl compared to opioid-free anesthesia (OFA) technique using ICNB and acetaminophen.
Methods
We conducted a prospective, single-blind, randomized comparative study over 18 months between January 2021 and June 2022 in Safdarjung Hospital, New Delhi after institutional ethical clearance. The study sample size was calculated by referring to a study by Kundal et al.17 The effect size was calculated as Cohen’s d. Alpha level was taken as 0.05 and the power of the study was taken as 0.9. Sample attrition was assumed to be 30% for each arm. The sample size was estimated using these parameters for an independent, two-tailed, t-test using the ‘pwr’ package in R Programming Language. The calculation returned a sample size of nine participants per arm with a total sample size of 18 participants. We included 60 participants in our study. We included full-term neonates with type C TEF18,19 undergoing surgical repair for fistula who were hemodynamically stable and not on ventilatory support. Preterm babies and neonates with other known congenital anomalies (including VACTERL vertebral defects, anal malformations, cardiac anomalies, TEF, radial/renal anomalies, and limb anomalies associations, etc.) or intubated neonates on ventilatory support and those whose parents/guardian refused to participate were excluded from the study.
The primary objective was to assess the extubation time and the rate of on-table extubation in neonates undergoing TEF repair. The secondary objective was to assess and compare the post-operative pain scores between opioid and OFA techniques.
All neonates underwent detailed preanesthetic evaluation. The nature of the study was explained to the parents and informed consent was obtained from them. A total of 60 neonates undergoing TEF repair were randomly allocated to two groups of 30 each using a computer-generated random number function. The child was taken to a prewarmed operation theatre and routine monitoring including heart rate, respiratory rate, five-lead electrocardiogram, blood pressure, temperature, and oxygen saturation were initiated. Neonates in group O were given fentanyl injection at 1 µg/kg IV loading dose with acetaminophen injection IV at 7.5 mg/kg. A bolus of fentanyl injection 0.25 µg/kg was given if necessary. In group NO, neonates were given pre-surgical local infiltration with 0.25% bupivacaine followed by ICNB with 0.5% bupivacaine administered by the surgeon in 2–3 intercostal spaces along with acetaminophen injection IV at 7.5 mg/kg. The airway was secured with an endotracheal tube of appropriate size. Location of fistula was assessed by fiberoptic bronchoscopy. End tidal CO2 and age-adjusted minimum alveolar concentration was monitored. GA was maintained with sevoflurane and atracurium injection IV. At the end of surgery, reversal agents were administered as appropriate. The extubation time was measured as the time taken from end of the skin suture to the tracheal tube removal. Vital parameters were monitored during the procedure. The operating surgical team was the same in both groups.
In the postoperative period, the patient’s pain score was checked and noted as per Neonatal Infant Pain Scale (NIPS)20 at every 15 minutes for the first hour, every 30 mins for the next two hours, and then hourly for a total of six hours after extubation or until the NIPS score was four [Figure 1]. The surgeon administering the ICNB is shown in Figure 2.
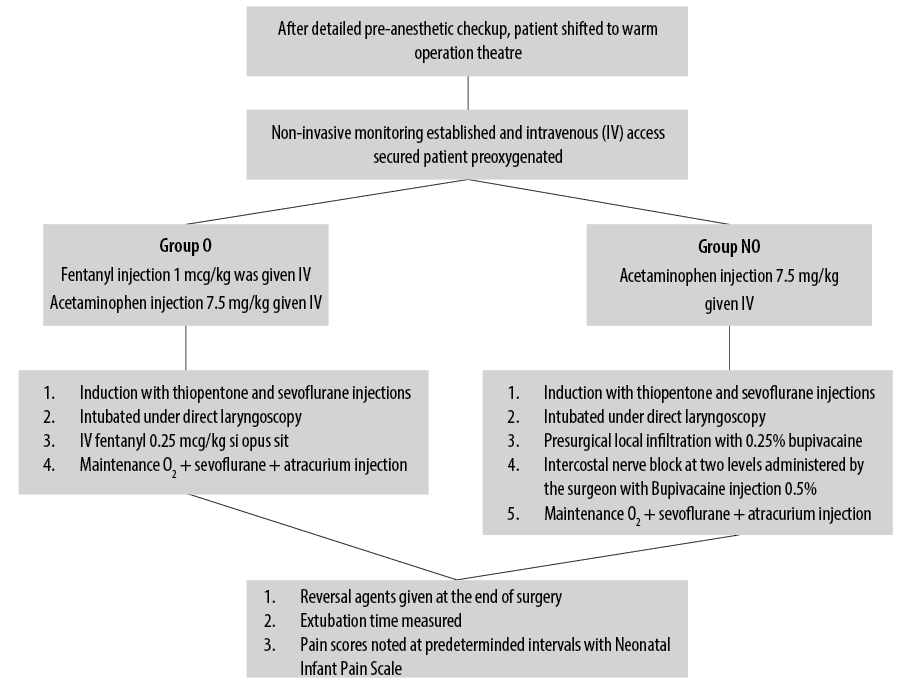 Figure 1: Flow chart describing the study method.
Figure 1: Flow chart describing the study method.
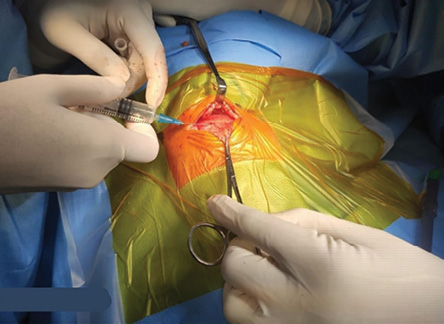 Figure 2: Surgeon administering intercostal nerve block with bupivacaine.
Figure 2: Surgeon administering intercostal nerve block with bupivacaine.
The collected data was transformed into variables, coded, and then entered into Microsoft Excel sheet. The data was analyzed and statistically evaluated using SPSS (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) The data normality was checked by using Shapiro-Wilk test. Quantitative data was expressed in mean and SD while qualitative data was expressed in percentage. The cases in which the data was not normal, we used nonparametric tests. The differences between the proportions were tested using the chi-square test and Fisher’s exact test. A p-value < 0.05 was considered statistically significant.
Results
Among the 60 neonates enrolled in the study, in both groups’ the majority were females (58.3%, n = 35). The mean age of the neonates was 2.2±1.6 days. The mean age in groups O and NO were 2.9±1.9 and 2.0±1.1 day, respectively. There was no statistically significant difference between the two groups in terms of gender distribution (p = 1.000) and mean age group (p = 0.088). The mean weight of the neonates group was 2.4±0.4 kg. The mean weight in group O was 2.4±0.4 kg and 2.4±0.4 kg in group NO. The mean weight of the two groups were statistically comparable but not significant (p = 0.460). Demographic variables of both groups are compared in Table 1. The mean operative time was 152.8±40.5 mins and was comparable in both groups.
Table 1: Demographic variables of the study groups.
|
Gender, n (%)
|
|
|
|
|
Male
|
13 (43.3)
|
12 (40.0)
|
1.000
|
|
Female
|
17 (56.7)
|
18 (60.0)
|
|
|
Age, days
|
2.5 ± 1.9
|
2.0 ± 1.1
|
0.088
|
The results are depicted as mean ± SD or number (%) unless otherwise stated.
Among the 60 neonates, 76.7% (n = 46) got extubated on table while 23.3% (n = 14) did not. In group O, 60.0% (n = 18) were extubated, while in group NO, 93.3% (n = 28) were extubated on table. On-table extubation between group O and group NO was statistically significant (p = 0.002) [Table 2].
Table 2: On-table extubation rate and extubation time in group O versus group NO.
|
On table extubation
|
|
|
|
|
Extubated
|
18 (60.0)
|
28 (93.3)
|
0.002
|
|
Not extubated
|
12 (40.0)
|
2 (6.7)
|
|
The results are depicted as mean ± SD or number (%) unless otherwise stated.
In our study, amongst the neonates who were extubated on table, the mean extubation time in group O was 16.0 mins 45.0 secs±8.0 mins 5.0 secs and in group NO was 9.0 mins 40.0 secs±3.0 mins 3.0 secs. The difference in on-table extubation time between group O and group NO was statistically significant (p < 0.001). Extubation time among the two groups compared for every five-minute interval is shown in Figure 3. NIPS was 1.8±0.8 among group NO and 2.5±1.1 among group O at 90 minutes which was statistically significant (p = 0.010). NIPS comparison between the two groups is shown in Table 3 and Figure 4.
Table 3: Neonatal Infant Pain Scale in group O versus group NO.
|
15
|
0.9 ± 0.9
|
1.0 ± 1.3
|
0.440
|
|
30
|
0.8 ± 0.9
|
0.6 ± 0.9
|
0.310
|
|
45
|
1.1 ± 1.1
|
0.9 ± 0.8
|
0.270
|
|
60
|
1.7 ± 0.9
|
1.5 ± 1.0
|
0.300
|
|
90
|
2.5 ± 1.1
|
1.8 ± 0.8
|
0.010
|
|
120
|
2.7 ± 1.0
|
2.5 ± 0.9
|
0.270
|
|
150
|
3.5 ± 0.7
|
3.3 ± 0.6
|
0.200
|
The results are depicted as mean ± SD or number (%) unless otherwise stated.
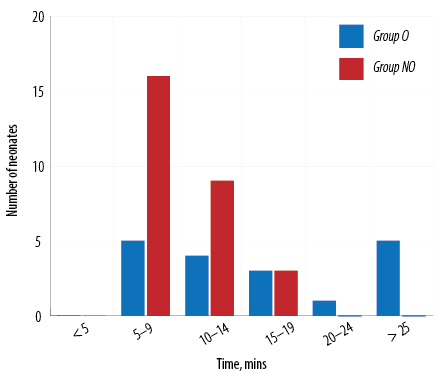 Figure 3: Comparing extubation time in group O versus group NO.
Figure 3: Comparing extubation time in group O versus group NO.
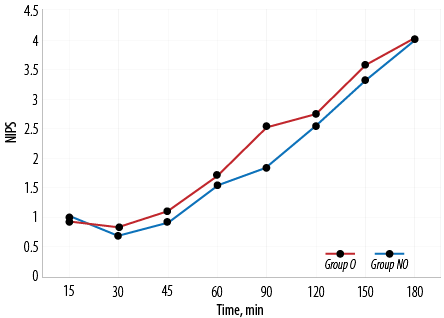 Figure 4: Neonatal Infant Pain Scale (NIPS) in group O versus group NO.
Figure 4: Neonatal Infant Pain Scale (NIPS) in group O versus group NO.
Discussion
Multiple modes of analgesia are available for TEF repair surgeries, including systemic analgesics like opioids and acetaminophen, opioid-free RA techniques, or a combination of these. This study was done to assess if OFA techniques can reduce the extubation time in neonates undergoing TEF repair while providing adequate analgesia.
In our study, the mean age of the neonates was 2.2±1.6 days. In a similar study, 30 neonates who underwent TEF were two days old at the time of surgery.21 In our study, delay in diagnosis postnatally and absence of antenatal diagnosis were some of the reasons the surgery was not done within 24 hours. The mean weight of the neonates was 2.4±0.4 kg. Thus, most babies enrolled for this study were > 2000 g excluding very low birth weight, a factor that delays extubation.
We found that 93.3% of neonates in group NO got extubated on table compared to only 60.0% in group O. This finding is of great clinical significance as, in resource-limited setups with a limited number of ventilators and trained personnel, the need for postoperative mechanical ventilation and the associated care can be reduced. In the single-blinded randomized control study by Nagappa et al,21 comparing opioids to OFA in 30 neonates undergoing TEF repair, 60% of the OFA group, compared to 20% of the opioid group were extubated on table. A retrospective study of 48 cases of gastroschisis undergoing repair, there was a significant difference in the need for postoperative ventilation in the OFA group (23%) compared to opioid-based analgesia (88%).22 In another retrospective study of 82 cases undergoing gastroschisis repair, only 14.3% needed postoperative mechanical ventilation compared to 40% of neonates requiring postoperative mechanical ventilation in the opioid group.17 This corroborates our finding that OFA is superior to opioid-based modes of analgesia for early extubation and reduced need for postoperative ventilation.
Amongst the neonates who got extubated on table, the mean extubation time in group O was 16.0 mins 45.0 secs±8.0 mins 5.0 secs and in group NO was 9.0 mins 40.0 secs±3.0 mins 3.0 secs. There was a statistically significant difference in the on-table extubation time between group O and group NO. Nagappa et al,21 found that the opioid group had significantly longer intubation periods than the non-opioid group.
Postoperative pain was assessed in both groups at predetermined times using NIPS. We found no statistically significant difference between the pain scores in the opioid and non-opioid groups except at 90 mins where the non-opioid group had a significantly lower pain score (1.8±0.8) compared to opioid group (2.5±1.1). This was of no clinical significance as the analgesic bolus was to be repeated once the pain score is four. Nagappa et al,21 found statistically significant differences in the mean NIPS at 30, 60, 90, 120 150, and 240 min intervals between group fentanyl and group epidural block, with RA technique providing better analgesia. This difference in postoperative analgesia could be because ICNB is only given at the beginning of the surgery in our study. We used ICNB, which is a simple technique not requiring any specific equipment, as it is easy to administer and has less complications. With pain assessment at frequent intervals and top-up given based on pain score, postoperative pain was managed with judicious use of analgesics. Despite extensive literature search, there are not many studies comparing postoperative analgesia between opioid and non-opioid techniques in TEF surgeries.
Our study has some limitations. Preterm neonates or neonates with other associated congenital anomalies were not included and factors other than opioid use could contribute to delayed extubation like preoperative chest condition.
We did not assess the duration of surgery and hemodynamic parameters in the study. NIPS, while easy to assess, is subjective and there could be inter-observer variability.
Conclusion
Neonates undergoing major surgery can receive regional anesthesia with faster extubation and minimal complications. There are also benefits related to the decreased need for muscle relaxants, opioid analgesics, and postoperative ventilator support. This study takes one more step towards bridging the gap in data on OFA in neonates.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. de Jong EM, Felix JF, de Klein A, Tibboel D. Etiology of esophageal atresia and tracheoesophageal fistula: “mind the gap”. Curr Gastroenterol Rep 2010 Jun;12(3):215-222.
- 2. Al-Rawi O, Booker PD. Oesophageal atresia and tracheo-oesophageal fistula. Contin Educ Anaesth Crit Care Pain 2007;7(1):15-19.
- 3. Morton NS, Fairgrieve R, Moores A, Wallace E. Anesthesia for the full-term and ex-premature infant. Gregory’s Pediatric Anesthesia 2020:533.
- 4. Edelman B, Selvaraj BJ, Joshi M, Patil U, Yarmush J. Anesthesia practice: review of perioperative management of H-type tracheoesophageal fistula. Anesthesiol Res Pract 2019 Nov;2019:8621801.
- 5. Charki S, Priyadarashini M, Hadalgi L, Agarwal S, Kulkarni T, Loni R, et al. Experience of tracheo-esophageal fistula in neonates in a tertiary care center - case series. J Clin Neonatol 2019;8(2):71.
- 6. Chi TL, Mirsky DM, Bello JA, Ferson DZ. Airway imaging: principles and practical guide. Benumof and Hagberg’s Airway Management 2013;21-75.
- 7. Khan S, Matta SR. Congenital anomalies. In: Nelson textbook of pediatrics. 2019. p. 1930.
- 8. Sfeir R, Rousseau V, Bonnard A, Gelas T, Aumar M, Panait N, et al. Risk factors of early mortality and morbidity in esophageal atresia with distal tracheoesophageal fistula: a population-based cohort study. J Pediatr 2021 Jul;234:99-105.
- 9. Knottenbelt G, Costi D, Stephens P, Beringer R, Davidson A. An audit of anesthetic management and complications of tracheo-esophageal fistula and esophageal atresia repair. Paediatr Anaesth 2012 Mar;22(3):268-274.
- 10. Kovar L, Weber A, Zemlin M, Kohl Y, Bals R, Meibohm B, et al. Physiologically-based pharmacokinetic (PBPK) modeling providing insights into fentanyl pharmacokinetics in adults and pediatric patients. Pharmaceutics 2020 Sep;12(10):1-21.
- 11. Koehntop DE, Rodman JH, Brundage DM, Hegland MG, Buckley JJ. Pharmacokinetics of fentanyl in neonates. Anesth Analg 1986 Mar;65(3):227-232.
- 12. Pacifici GM, Allegaert K. Clinical pharmacology of paracetamol in neonates: a review. Curr Ther Res Clin Exp 2014 Dec;77:24-30.
- 13. Kopacz DJ, Thompson GE. Intercostal blocks for thoracic and abdominal surgery. Tech Reg Anesth Pain Manage 1998;2(1):25-29.
- 14. Morton NS. Local and regional anaesthesia in infants. Continuing Education in Anaesthesia Critical Care and Pain 2004;4(5):148-151.
- 15. Kaye AD, Urman RD, Rappaport Y, Siddaiah H, Cornett EM, Belani K, et al. Multimodal analgesia as an essential part of enhanced recovery protocols in the ambulatory settings. J Anaesthesiol Clin Pharmacol 2019 Apr;35(Suppl 1):S40-S45.
- 16. Brindle ME, McDiarmid C, Short K, Miller K, MacRobie A, Lam JY, et al. Consensus guidelines for perioperative care in neonatal intestinal surgery: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg 2020 Aug;44(8):2482-2492.
- 17. Kundal R, Dogra N, Kundal VK. Anaesthetic considerations in gastroschisis repair at a tertiary care hospital. Sri Lanka J Anaesthesiol 2019;27(2):115-119.
- 18. Broemling N, Campbell F. Anesthetic management of congenital tracheoesophageal fistula. Paediatr Anaesth 2011 Nov;21(11):1092-1099.
- 19. Lee S. Basic knowledge of tracheoesophageal fistula and esophageal atresia. Adv Neonatal Care 2018 Feb;18(1):14-21.
- 20. Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Netw 1993 Sep;12(6):59-66.
- 21. Nagappa S, Kalappa S, Vijayakumar HN, Nethra HN. Comparison of the effectiveness of intravenous fentanyl versus caudal epidural in neonates undergoing tracheoesophageal fistula surgeries. Saudi J Anaesth 2022;16(2):182-187.
- 22. Raghavan M, Montgomerie J. Anesthetic management of gastrochisis–a review of our practice over the past 5 years. Paediatr Anaesth 2008 Nov;18(11):1055-1059.