The Shigella genus, a member of the Enterobacteriaceae family, is the invasive pathogen and etiologic agent of human shigellosis, which causes watery or mucoid/bloody diarrhea following the host inflammatory responses. This gram-negative genus comprises Shigella dysenteriae, Shigella flexneri, Shigella sonnei, and Shigella boydii serogroups, also known as Shigella subgroups A, B, C, and D, respectively.1–5 Shigella infections are generally transmitted through the fecal-oral route. Low hygiene is considered the main cause of its community transmission.6 This bacterium is the second cause of mortality via diarrhea and is globally responsible for approximately 212 000 death per annum, mainly among under-five-year-old children.7 The prevalence of members of the genus Shigella varies in different geographical regions. S. flexneri serotypes 2a, 1b, 3a, 4a, and 6 accounts for most cases of shigellosis in developing countries.8-10Other S. flexneri serotypes, almost 23 serotypes in total, are reportedly associated with various human diseases.11-13
In general, the pathogenic mechanism of shigellosis is common among all the serotypes, but there are some serotype-specific pathogenic features.14 For instance, only S. flexneri serotype 2a strains have the ability to exploit the immune modulating activity of protease involved in intestinal colonization (Pic) and impair leukocyte trafficking and migration. It has been postulated that this virulence factor is responsible for the dominance of S. flexneri serotype 2a in Shigella outbreaks.15-16
Serotype conversions and the emergence of new serotypes such as serotype Z in Shigella species are mediated by their ability to modify O-antigen to evade the host’s immune system.17 This information, mainly provided by sequencing and phylogenic analysis studies, will be discussed further in this article. Nontypeable Shigella strains, which cannot be recognized by commercial antisera and are often indistinguishable from other serotypes by common DNA-based molecular techniques, have been reported in several studies.11-18
While it is not clear why S. flexneri undergoes serotype conversion, it seems to enable S. flexneri to evade host defense responses.19 Studies also show that genes encoding glucosylation can provide evolutionary benefits including facilitating bacterial cell-to-cell spreading and resistance to environmental conditions such as the stomach’s low-pH environment.1,17
All these suggest that the unique characteristics of each serotype, depending on environmental conditions, can lead to the selection of a specific serotype or serotypes in a geographic population. This emphasizes the importance of conducting epidemiological surveillance tailor-made to geographical region/population. In this review, we shall provide an overview of biochemical and molecular processes involving the development of S. flexneri serotypes and molecular analysis of serotype conversion. The review also provides evidence of serotype conversion in epidemiological surveillance and its importance. We also discuss correlations between serotype conversion and acquired drug resistance or pathogenicity traits, in addition to the new-generation molecular methods for differentiating various S. flexneri serotypes.
Methodology and research strategies
Studies from 1995 to 2021 that addressed the topics of S. flexneri serotypes, O-antigen structure, and serotype conversion were accessed from PubMed, Web of Science, Scopus, ScienceDirect, and Springer literature databases. Several terms and keywords of ‘Shigella flexneri serotyping’, ‘O-antigen structure’, ‘O-antigen biosynthesis’, ‘O-antigen glucosylation’, ‘phosphoethanolamine transferase gene’, ‘phosphoethanolamine modification’, ‘serotype conversion’, ‘Shigella flexneri serovars’, ‘serotype-converting bacteriophages’, ‘evolutionary advantage’, and ‘molecular typing’ were used to identify potentially relevant studies. Serotype conversion, O-antigen modification in S. flexneri, serotyping approaches, and immunogenicity data were included in this study. Studies before 1995, studies containing discrepant data, case reports, congress abstracts, letters to the editor, and studies that were limited to antimicrobial resistance, pathogenicity, and outbreaks were excluded from the study. A total of 105 qualified articles were selected to be included in this review.
The key information extracted from the selected 105 scientific articles is summarized and reviewed below.
O-antigen diversity of S. flexneri
Due to the lack of H antigen in Shigella species, the serotyping of this bacterium is limited to O-antigen. O-antigen is a component of the outer membrane lipopolysaccharide of the bacterium, which plays a critical role in the induction of host immune response.8,9 All, except serotype 6, share common repeating units of tetrasaccharide in their polysaccharide backbone, which is composed of three L-rhamnose residues (rhamnose (Rha) I, RhaII, RhaIII) and one residue of 2-acetamido-2-deoxy-d-glucose (N-acetylglucosamine (GlcNAc))3 [Figure 1].
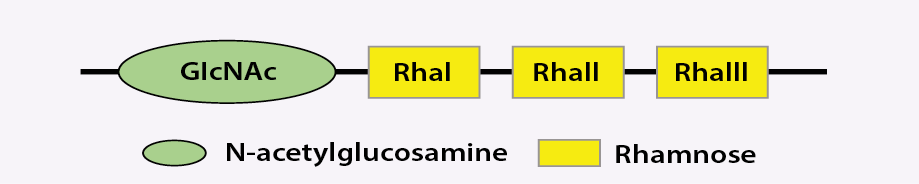 RhaI, Rha, and RhaIII: L-rhamnose residues; GlcNAc: 2-acetamido-2-deoxy-d-glucose.
RhaI, Rha, and RhaIII: L-rhamnose residues; GlcNAc: 2-acetamido-2-deoxy-d-glucose.
Figure 1: Schematic structure of common Shigella flexneri tetrasaccharide backbone (except serotype 6).
Serotype Y of S. flexneri has the basic O-antigen with single group 3:4 antigenic determinants, which are encoded by the chromosomal rfc. Any modification of O-antigen structure, such as the addition of glucosyl, O-acetyl, or phosphoethanolamine (pEtN), to different sugars in the tetrasaccharide backbone, is involved in serotype conversion of S. flexneri.8,20-22 The classification of S. flexneri serotypes is based on the placement of glucosyl, O-acetyl, or pEtN on different sugars with different bonds on tetrasaccharide backbone, which engender different types (I, II, III, IV, V, IC, or VII) and group-specific 6–10 antigenic determinants in different serotypes.18,21 It should be noted that although O-antigen is type specific in each serotype, O-antigen groups exist among serotypes with a different type of O-antigen.22,23 Glucosylation of O-antigen monosaccharides including RhaI, RhaII, or GlcNAc at various positions and residues will engender different antigenic types or groups.3,23,24 O-acetylation occurs on Rha and GlcNAc, which is based on the position and residues that are subjected to modification, and can provide different types and groups of O-antigen (e.g., groups 6, 7, 8, and type III).23
The addition of pEtN on RhaIII and/or RhaII was reported in 4av, Xv, Yv, and Yv1 serotypes and the ‘v’ letter is useful for all serotypes which carry pEtN modification.23
Serotype 6 of S. flexneri is genetically different and its O-antigen consists of 2 L-rhamnose, D-galacturonic acid, and GlcNAc, which is substituted by GlcNAc in other types of S. flexneri O-antigens [Figure 2]. This change in the O-antigen structure is unique and is not reported in other serotypes, currently.25
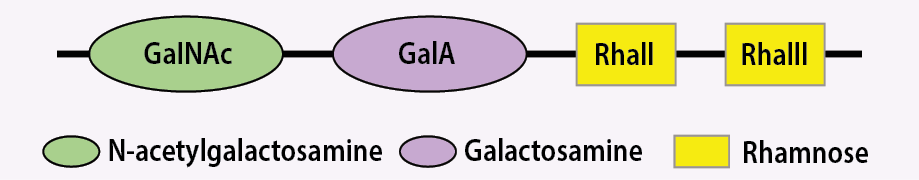 Figure 2: Schematic structure of common Shigella flexneri tetrasaccharide backbone of serotype 6.
Figure 2: Schematic structure of common Shigella flexneri tetrasaccharide backbone of serotype 6.
Genetic basis of serotype conversion
Serotype-converting bacteriophages are participating in Shigella O-antigen modification.26-28 The reason why some bacteriophages carry O-antigen modification genes is that they use O-antigenic polysaccharide chains as receptors for adsorption.8,21,29
O-antigen modification can protect bacteriophages by inhibiting other homologs’ bacteriophage adsorption. It is also advantageous to the bacteria by enabling them to evade the host's immune response. This modification occurs before O-antigen polysaccharide backbone transfers to the lipid A core.30 These bacteriophages induce glucosylation through proteins encoded by glycosyltransferase (gtr) operon in the bacterial chromosome. Bacteriophages involved in this process include SfI, SfII, SfIV, SfV, and SfX. Although these phages belong to various virus families, they share a similar position for glucosyl transferase genes.8,31
The genes responsible for glucosylation of polysaccharide backbone include gtrA, gtrB, and the serotype-specific gtr which are located in a single cassette known as a gtr cluster. GtrB plays an important role in the synthesis of undecaprenyl phosphate-β-glucose (UndP-β-Glc) from UDP-α-Glc, and gtrA facilitates translocating UndP-β-Glc from the cytoplasm into periplasm, where O-antigen modification occurs. Unlike gtrA and gtrB which are highly conserved, gtr (type) encodes serotype-specific glucosyltransferases—GtrI, GtrII, GtrIV, GtrV, GtrVII (formerly GtrIc), and GtrX—that cause glucosyl group attachment to specific sugar units.31 As previously discussed, the addition of an O-acetyl group to RhaI at position 2 (2-O-acetylation), RhaIII at position 3 or 4 (3/4-O-acetylation), or GlcNAc constitutes another form of O-antigen modification, which is mediated by different genes and responsible for the generation of different serotypes. The gene responsible for acetylation in 3a, 3b, and 4b serotypes is named oac and encoded by sf6 bacteriophage, which causes serotype variation by adding an acetyl group to RhaI at position 2 (2-O-acetylation). On the contrary, acetylation of RhaI at position 2 (2-O-acetylation) of 1b and 7b serotypes is done by an oacA variant named oac1b, which is encoded by sf6 bacteriophage. It is important to note that sf6 bacteriophage is not the only transferable element responsible for acetylation and that phages other than sf6 can also be responsible for this process.23,32 Unlike other serotypes, 3/4-Oacetylation of RhaIII in serotype 6 is mediated by oacB homolog called oacC, which is encoded by a bacteriophage structure located in another place on the chromosome that is different from oacB.33,34 The last-mentioned modification is related to GlcNAc acetylation.
This modification is conducted by oacD, which is located on serotype-converting bacteriophage sfII. Integration of sfII into the host chromosome by lysogeny is responsible for this modification, which occurs in 2, 3a1, X1, Y1, Y2, and Yv1 serotypes. SfII bacteriophage encodes both acetyltransferase and glucosyltransferase genes, but their functions are independent of each other. So that the gtr locus, which is responsible for glucosylation, is dysfunctional in 3a, Y, and Yv serotypes, and 2a is the only serotype in which both the enzyme are active.34
The polymorphic gene lpt-O is located on pSFxv-2 or pSFyv-2 plasmids. Both pSFxv-2 and pSFyv-2 can mobilize among varied serotypes of S. flexneri and cause unnatural O-factor IV-1-positive ‘variants’ (v), including 4av, Xv, Yv, and Yv1 serotypes. These serotypes are engendered by the addition of the pEtN group to position 3 of both RhaII or/and RhaIII. Although RhaII is phosphorylated in both 4av and Yv serotypes, the Xv serotype carries the pEtN group on RhaIII. Evidence suggests that the genes responsible for this modification pattern are distinct and have been nominated as lpt-ORII and lpt-ORIII through the phosphorylation of RhaII and/or RhaIII, respectively. Studies also showed that acetylation is affected by the degree of phosphorylation.35 Though phosphorylation of both RhaII and RhaIII hinders O-acetylation, phosphorylation of RhaIII by itself does not. Two different genes (lpt-ORII and lpt-ORIII) are responsible for pEtN modification. Studies show that the difference between these genes is probably correlated to 11 base changes and seven amino acid changes in order to be adapted to the O-antigen structures based on the serotype.35,36 For instance, lpt-ORIII comfortably positions pEtN onto RhaIII in 4a serotype as this position is not occupied, while RhaIII in X serotype is occupied by glucosyl group. Therefore, selective pressure causes lpt-ORII to choose RhaII in order to add the pEtN group to RhaII instead of RhaIII. Other serotypes that are affected by this modification include Yv and Yv1, which acquire the pEtN group from either lpt-ORII or lpt-ORIII origin. The lpt-ORII generates Yv strains from Y or Xv, and lpt-ORIII is determined to convert serotype 2 into serotype Yv1.21,23
Molecular aspects of S. flexneri serotype conversion
Serotype diversity in S. flexneri evolves as a result of horizontal transfer of transposable elements, which encode O-antigen modification enzymes that in turn facilitate serotype conversions among the various strains.18,36 For example, serotype conversion by glucosylation genes of bacteriophages can convert Y serotype to 1a, 2a, 4a, 5a, and X serotypes, respectively.23 Studies have been conducted on the mechanisms in which glucosylation causes serotype conversion. The study on SfX lysogenic bacteriophage proposed that after synthesis of UndP-β-Glc from UDP-α-Glc, the former flips to the periplasmic side of the cytoplasmic membrane either by gtrA alone or by gtrA associated with gtr (type). Then the glucosyl group transfers to the growing polysaccharide chain by gtr (type), which acts as a specific glucosyl transferase. As the antigen-carrier lipid is still intact after glucosylation of polysaccharide backbone by gtrX, polymerization and transfer can be continued by Wzy and WaaL (two proteins responsible for polymerization and ligation) on the growing polysaccharide chain. The lipid carrier associated with this glucosylation process uses similar biosynthesis pathway(s) with many other bacterial outer-membrane structures. The regeneration of these molecules is crucial to the bacterial cell function, as the shortage of lipid carriers on the bacterial cytoplasmic membrane may be associated with bacterial lysis.30
The remarkable point is that if S. flexneri is affected by several bacteriophages, serotype diversity will increase. However, each bacteriophage has a specific host range; for example, SfI is specific for serotypes X and Y; SfII is specific for serotypes 3a, 5a, and Y; SfIV is specific for serotypes 1a, 1b, 1c, and X; and SfV is specific for serotypes 1a, 1b, 2a, 2b, 3b, 4b, and Y.37
Mutations encoded by bacteriophages may cause inactivation of gtr locus in S. flexneri strains, which can lead to serotype reversion or conversion. Surveillance studies showed some strains that carried defective forms of glucosylation genes, which had evolved from the wild-type genes. These mutations are responsible for the conversion of some types of serotypes.
The same serotypes may have quite different origins. For instance, Yv and Yv1 subtypes could be the results of several other serotypes at their origins. Accordingly, they could be the result of either the acquisition of an opt-carrying plasmid of Y serotype, inactivation of a gtr gene of Xv serotype, or a combination of the latter two occurrences of 2a serotype.21 In addition, some serotypes may be generated from two distinct glucosylation genes. The study conducted by Stagg et al,6 shows that out of two glucosyl groups that serotype 7 possesses, the first one is mediated by the same gtr cluster inside SfI prophage, which encodes glucosyl group in this serotype, but the second glucosyl group is a result of gtrIc glucosyltransferase. As gtrIc glucosyltransferase is integrated at a dissimilar place adjacent to the conserved yejO in the bacterial chromosome, it is possible that gtrI and gtrIc clusters are attained from different bacteriophages.
Serotype conversion in epidemiological surveillance
There is some evidence that serotype conversion is the key selective pressure for the emergence of the S. flexneri epidemic clones and is critical for the pathogen’s evolution.38 Evidence also suggests that serotype conversion of S. flexneri occurs not only in the environment, but within the human host as well.39,40 The emergence of new S. flexneri serotypes is a major healthcare problem since some may potentially evade the host's immune response and are undetectable by conventional methods.11,17
As previously mentioned, predominant serotypes may be associated with the development of novel serotypes that can provide survival advantages to S. flexneri in order to be dominant in the future. For example, 4s serotype, which appeared in Henan Province, China in 2010, is supposed to have been derived from Xv serotype even though molecular analysis shows that this serotype has evolved from 2a, 2b, 2c, and Xv serotypes. Yv serotype is another novel serotype that is thought to have evolved from Y, Xv, and 2a in different manners, according to its ancestor.17,18,21,41 Serotype conversion is not limited to the emergence of new serotypes and can occur among common serotypes. A study in China suggests that the common 1a serotype may have changed to 2a in the three decades from 1972 to 2010.42 Another study showed that the 2a serotype which was the most prevalent serotype from 2000 to 2001 in Henan Province, China, was later replaced by a novel serotype, Xv, from 2002 to 2006.5 Frequent serotype switching in a clone of S. flexneri was determined as the cause of this replacement.5
Importance of serotype conversion
The capacity for many serotype conversions in S. flexneri plays an essential role in the emergence and distribution of distinct serotypes of S. flexneri in different human populations.17 In general, serotype conversion is engendered becuase of genetic modification, which can participate in the development of serotypes that not encountered earlier in a population. This emergence is clinically important since protective immunity to some serotypes need not confer cross-protection against others. Serotype switching may be the main reason why S. flexneri infection is still persisting despite the global improvement in hygiene.5 Moreover, this switching may provide evolutionary advantages to some serotypes, with a continued threat to public health.5
Serotype conversion-mediated antibiotic resistance and virulence traits
S. flexneri, due to its capacity for serotype conversion, could confer differences in its pathogenesis.43 Microevolution of each strain, through distinct genetic events linked to O-antigen modification and the acquisition of resistance genes, could lead to the emergence of a new strain with different pathogenic capacities. A study on S. flexneri ST91 serotype Xv showed that the strain was related to serotype II, common in China.17 Multiple evolutionary events, including the acquisition of antibiotic resistance genes, had happened in this strain before the occurrence of the serotype conversion event. This phenomenon is not exclusive to serotype Xv but has been observed in others such as serotype 4s.17 It seems that new serotypes are more likely to evolve from common serotypes in a region, which may eventually result in proliferation of clinically important S. flexneri serotypes in that region.38
Serotype conversion can affect S. flexneri pathogenicity. A study reported serotype S. flexneri 1c occurring as a result of the acquisition of gtrI, gtrIc, and oacB genes via three distinct bacteriophages, which were related to massive DNA deletion, giving a possible explanation for the genetic diversity of this strain. Since the protective immune response to S. flexneri mainly targets the O-antigen, extensive diversity of O-antigen modification in serotype 1c could facilitate S. flexneri escaping from immune cells, which affects its pathogenesis.44 In a study by Nie et al,45 it was supposed that genetic events (such as inversion, translocation, deletion, and acquisition) could lead to diversity in serotype conversion region, possibly accompanied by loss or acquisition of other virulence loci including Shigella pathogenicity islands 1 and 2. Some of these changes could increase expression levels of virulence genes among the newly-evolved serotypes.45
Cell invasion of S. flexneri is mediated by the type three secretion system (T3SS).46 The study conducted by West et al,47 showed glycosylation of O-antigen promotes Shigella invasion by affecting the T3SS. Glucosylated O-antigen is more compact and shorter than non-glucosylated antigen, which allows the proper function of T3SS.47 Additionally, a more compact and denser O-antigen facilitates interaction between O-antigen molecules, contributing to the stabilization of the outer membrane.48 The length of O-antigen in Shigella exerts some effects on its function. In a study by Morona et al,49 a strong link between chain length of O-antigen in S. flexneri serotype 2a and its effect on the regulation of the IcsA function was established. S. flexneri serotype 2a encodes bimodal O-antigen, including S-type O-antigen (short-type) and VL-type O-antigen (very long-type).49 The S-type O-antigen contributes to IcsA mediated motility of S. flexneri, while VL-type O-antigen may mask IcsA and cause resistance to direct complement-mediated serum killing.49 In addition, VL-type O-antigen is proposed to be important for resistance to direct complement-mediated serum killing in S. flexneri 2a.50 The length of O-antigen chain is critical for invasion (adherence to and internalization) of S. flexneri serotype 2a to host macrophages and epithelial cells.51,52 In vitro expression of different O-antigen modals is growth-phase-dependent; however, further studies are needed to characterize mediators that are involved in structural changes of O-antigen in host tissue.51
Lipopolysaccharide variation is also an important strategy used by bacteria to adapt to environmental conditions. The presence of an S-type O-antigen is not only required for the cell-to-cell spreading of S. flexneri, but it also causes optimal acid resistance as a VL-type O-antigen that masks membrane protein, which is associated with acid resistance.1 There is no study to describe other modifications, like acetylation, and their roles in the virulence of Shigella serotypes.
New-generation molecular methods
The main drawback of S. flexneri serotypes nomination is that there is no standard definition of S. flexneri serotypes, so according to the emergence of a variety of new serotypes, there is a necessity for a new revision of S. flexneri nomenclature.53Traditional S. flexneri serotyping was developed based on the slide agglutination method using polyclonal and monovalent antisera.53 Due to the cross-reaction of polyclonal antisera, monoclonal antibodies are needed to differentiate Shigella serotypes; however, there are some drawbacks since some strains do not express O-antigen and are indistinguishable from serological tests.53 Moreover, serological identification is time-consuming and requires a variety of antisera that are unavailable in most laboratories. Newer molecular serotyping methods, such as polymerase chain reaction (PCR)-restriction fragment length polymorphism, multiplex PCR, and microarray are capable of providing reliable results more quickly.4,54-58The extensively used multiplex PCR method is based on the genes responsible for both O-antigen synthesis and modification. The O-antigen flippase gene (wzx) exists in all serotypes except serotype 6, so it can also function as a control for PCR. For serotype 6, it is better to use singleplex PCR for the wzx gene. Other genes that could be used in molecular serotyping include those relevant to glucosylation (gtr genes), acetylation (oac gene), and phosphorylation (opt gene). The association of these genes with distinct serotypes of Shigella is shown in Table 1.18,53,56,58,59 Although in some of the bacteria there are drawbacks for molecular serotyping methods compared with slide agglutination tests as the gold standard method, which are mainly caused based on the expression of inactive forms of the noted genes, there is a reasonable similarity between the slide agglutination method and multiplex PCR results among S. flexneri serotypes.18,59 This similarity has encouraged researchers to use this method for the serotyping of S. flexneri in laboratories.56,58
Table 1: Multiplex polymerase chain reaction patterns of different serotypes of Shigella flexneri.
|
1a
|
+
|
+
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
1b
|
+
|
+
|
-
|
-
|
-
|
-
|
-
|
+
|
-
|
-
|
|
7a(1c)
|
+
|
+
|
+
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
1d
|
+
|
+
|
-
|
-
|
-
|
-
|
+
|
-
|
-
|
-
|
|
2a
|
+
|
-
|
-
|
+
|
-
|
-
|
-
|
-
|
-
|
-
|
|
2b
|
+
|
-
|
-
|
+
|
-
|
-
|
+
|
-
|
-
|
-
|
|
3a
|
+
|
-
|
-
|
-
|
-
|
-
|
+
|
+
|
-
|
-
|
|
3b
|
+
|
-
|
-
|
-
|
-
|
-
|
-
|
+
|
-
|
-
|
|
4a
|
+
|
-
|
-
|
-
|
+
|
-
|
-
|
-
|
-
|
-
|
|
4b
|
+
|
-
|
-
|
-
|
+
|
-
|
-
|
+
|
-
|
-
|
|
4av(4c)
|
+
|
-
|
-
|
-
|
+
|
-
|
-
|
-
|
+
|
-
|
|
4S(z)
|
+
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
+
|
|
|
5a
|
+
|
-
|
-
|
-
|
-
|
+
|
-
|
±
|
-
|
-
|
|
5b
|
+
|
-
|
-
|
-
|
-
|
+
|
+
|
±
|
-
|
-
|
|
6
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
+
|
|
X
|
+
|
-
|
-
|
-
|
-
|
-
|
+
|
-
|
-
|
-
|
|
Xv
|
+
|
-
|
-
|
-
|
-
|
-
|
+
|
-
|
+
|
-
|
|
Y
|
+
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
+: having the gene; -: not having the gene.
Molecular methods also have drawbacks, since no multiplex PCR assay has been designed that can distinguish all the existing serotypes. For instance, none of the 4d, 4e, 6b, and 7b serotypes can be differentiated from other serotypes by the multiplex PCR method. This is also true with different subtypes (serotypes X and Y).18
Whole genome sequencing (WGS) analysis is a vigorous tool for serotyping purposes and also provides information about the phylogenic relationship between different S. flexneri serotypes. This method is helpful for surveying novel serotypes.59 WGS is at present too expensive for routine use in clinical laboratories. In addition, interpreting WGS results is quite difficult, though this has been partially solved by the new ‘ShigaTyper’ tool v2.0.2-0 (https://github.com/CFSAN-Biostatistics/shigatyper), which uses low-computing power to automate the workflow to accurately and rapidly determine Shigella serotypes using Illumina paired-end WGS reads.57
Clinical aspects of S. flexneri serotyping
In view of the low infectious dose and increasing levels of antibiotic resistance among Shigella species, prevention through vaccine development is a topic of ongoing research. Some serotypes share common group- and type-specific antigens which can provide cross-protection. Therefore, it is important to broaden our knowledge of serotype structures to better understand their common features to develop more efficient vaccines. Moreover, continuous surveillance can give more reliable data about circulating types which can help to better management of patients by designing more targeted vaccines.
Conclusion
Serotype diversity of S. flexneri evolves as a result of horizontal transfer of transposable elements which are responsible for serotype conversion among S. flexneri strains. As serotype conversion plays a key role in both the evolution and survival of S. flexneri infections, structural and epidemiological studies need to keep up with new evolving serotypes associated with human diseases. Detection of these serotypes by molecular methods could be an alternative technique for seroepidemiology and surveillance studies. Characterization of new serotypes could help us understand the mechanisms that these pathogens exploit to bypass the host immune system, as well as develop more efficient and population-specific vaccines.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Martinić M, Hoare A, Contreras I, Alvarez SA. Contribution of the lipopolysaccharide to resistance of Shigella flexneri 2a to extreme acidity. PLoS One 2011;6(10):e25557.
- 2. Baker S, The HC. Recent insights into Shigella. Curr Opin Infect Dis 2018 Oct;31(5):449-454.
- 3. Kenne L, Lindberg B, Petersson K, Katzenellenbogen E, Romanowska E. Structural studies of Shigella flexneri O-antigens. Eur J Biochem 1978 Nov;91(1):279-284.
- 4. Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Wang Q, et al. Structure and genetics of Shigella O antigens. FEMS Microbiol Rev 2008 Jul;32(4):627-653.
- 5. Ye C, Lan R, Xia S, Zhang J, Sun Q, Zhang S, et al. Emergence of a new multidrug-resistant serotype X variant in an epidemic clone of Shigella flexneri. J Clin Microbiol 2010 Feb;48(2):419-426.
- 6. Stagg RM, Tang S-S, Carlin NI, Talukder KA, Cam PD, Verma NK. A novel glucosyltransferase involved in O-antigen modification of Shigella flexneri serotype 1c. J Bacteriol 2009 Nov;191(21):6612-6617.
- 7. GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis 2018 Nov;18(11):1211-1228.
- 8. Allison GE, Verma NK. Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri. Trends Microbiol 2000 Jan;8(1):17-23.
- 9. Cui X, Yang C, Wang J, Liang B, Yi S, Li H, et al. Antimicrobial resistance of Shigella flexneri serotype 1b isolates in China. PLoS One 2015 Jun;10(6):e0129009.
- 10. Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 1999;77(8):651-666.
- 11. Das A, Mandal J. Extensive inter-strain diversity among clinical isolates of Shigella flexneri with reference to its serotype, virulence traits and plasmid incompatibility types, a study from south India over a 6-year period. Gut Pathog 2019 Jun;11(1):33.
- 12. Luo X, Sun Q, Lan R, Wang J, Li Z, Xia S, et al. Emergence of a novel Shigella flexneri serotype 1d in China. Diagn Microbiol Infect Dis 2012 Nov;74(3):316-319.
- 13. Talukder KA, Dutta DK, Safa A, Ansaruzzaman M, Hassan F, Alam K, et al. Altering trends in the dominance of Shigella flexneri serotypes and emergence of serologically atypical S. flexneri strains in Dhaka, Bangladesh. J Clin Microbiol 2001 Oct;39(10):3757-3759.
- 14. Barry EM, Pasetti MF, Sztein MB, Fasano A, Kotloff KL, Levine MM. Progress and pitfalls in Shigella vaccine research. Nat Rev Gastroenterol Hepatol 2013 Apr;10(4):245-255.
- 15. Mattock E, Blocker AJ. How do the virulence factors of Shigella work together to cause disease? Front Cell Infect Microbiol 2017 Mar;7:64.
- 16. Ruiz-Perez F, Wahid R, Faherty CS, Kolappaswamy K, Rodriguez L, Santiago A, et al. Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins. Proc Natl Acad Sci U S A 2011 Aug;108(31):12881-12886.
- 17. Yang C, Li P, Zhang X, Ma Q, Cui X, Li H, et al. Molecular characterization and analysis of high-level multidrug-resistance of Shigella flexneri serotype 4s strains from China. Sci Rep 2016 Jul;6:29124.
- 18. Shahnaij M, Latif HA, Azmi IJ, Amin MB, Luna SJ, Islam MA, et al. Characterization of a serologically atypical Shigella flexneri Z isolated from diarrheal patients in Bangladesh and a proposed serological scheme for Shigella flexneri. PLoS One 2018 Aug;13(8):e0202704.
- 19. George DT, Stephenson DP, Tran E, Morona R, Verma NK. Complete genome sequence of SfII, a serotype-converting bacteriophage of the highly prevalent shigella flexneri serotype 2a. Genome Announc 2013 Sep;1(5):e00626-13.
- 20. Carlin NI, Lindberg AA. Monoclonal antibodies specific for Shigella flexneri lipopolysaccharides: clones binding to type IV, V, and VI antigens, group 3,4 antigen, and an epitope common to all Shigella flexneri and Shigella dysenteriae type 1 stains. Infect Immun 1987 Jun;55(6):1412-1420.
- 21. Sun Q, Lan R, Wang J, Xia S, Wang Y, Wang Y, et al. Identification and characterization of a novel Shigella flexneri serotype Yv in China. PLoS One 2013 Jul;8(7):e70238.
- 22. van der Ploeg CA, Rogé AD, Bordagorría XL, de Urquiza MT, Castillo AB, Bruno SB. Design of two multiplex PCR assays for serotyping Shigella flexneri. Foodborne Pathog Dis 2018 Jan;15(1):33-38.
- 23. Knirel YA, Sun Q, Senchenkova SN, Perepelov AV, Shashkov AS, Xu J. O-antigen modifications providing antigenic diversity of Shigella flexneri and underlying genetic mechanisms. Biochemistry (Mosc) 2015 Jul;80(7):901-914.
- 24. Wang J, Lan R, Knirel YA, Luo X, Senchenkova SN, Shashkov AS, et al. Serological identification and prevalence of a novel O-antigen epitope linked to 3- and 4-O-acetylated rhamnose III of lipopolysaccharide in Shigella flexneri. J Clin Microbiol 2014 Jun;52(6):2033-2038.
- 25. Pupo GM, Lan R, Reeves PR. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc Natl Acad Sci U S A 2000 Sep;97(19):10567-10572.
- 26. Boyd JS. The antigenic structure of the mannitol-fermenting group of dysentery bacilli. J Hyg (Lond) 1938 Jul;38(4):477-499.
- 27. Ewing WH. Mannitol negative varieties of Shigella flexneri serotypes. J Immunol 1954 May;72(5):404-410.
- 28. Takita J. A new type of antigenic variation occurring in the Flexner group of dysentery bacilli. J Hyg (Lond) 1937 Apr;37(2):271-279.
- 29. Lindberg AA. Bacterial surface polysaccharides and phage adsorption. Surf Carbohydrates Prokaryotic Cell 1977:289-356.
- 30. Guan S, Bastin DA, Verma NK. Functional analysis of the O antigen glucosylation gene cluster of Shigella flexneri bacteriophage SfX. Microbiology (Reading) 1999 May;145(Pt 5):1263-1273.
- 31. Markine-Goriaynoff N, Gillet L, Van Etten JL, Korres H, Verma N, Vanderplasschen A. Glycosyltransferases encoded by viruses. Journal of General Virology 2004 Oct 1;85(10):2741-2754.
- 32. Sun Q, Lan R, Wang Y, Wang J, Xia S, Wang Y, et al. Identification of a divergent O-acetyltransferase gene oac 1b from Shigella flexneri serotype 1b strains. Emerg Microbes Infect 2012 Sep;1(9):e21.
- 33. Knirel YA, Wang J, Luo X, Senchenkova SN, Lan R, Shpirt AM, et al. Genetic and structural identification of an O-acyltransferase gene (oacC) responsible for the 3/4-O-acetylation on rhamnose III in Shigella flexneri serotype 6. BMC Microbiol 2014 Oct;14(1):266.
- 34. Sun Q, Knirel YA, Wang J, Luo X, Senchenkova SN, Lan R, et al. Serotype-converting bacteriophage SfII encodes an acyltransferase protein that mediates 6-O-acetylation of GlcNAc in Shigella flexneri O-antigens, conferring on the host a novel O-antigen epitope. J Bacteriol 2014 Oct;196(20):3656-3666.
- 35. Knirel YA, Lan R, Senchenkova SN, Wang J, Shashkov AS, Wang Y, et al. O-antigen structure of Shigella flexneri serotype Yv and effect of the lpt-O gene variation on phosphoethanolamine modification of S. flexneri O-antigens. Glycobiology 2013 Apr;23(4):475-485.
- 36. Sun Q, Knirel YA, Lan R, Wang J, Senchenkova SN, Shashkov AS, et al. Dissemination and serotype modification potential of pSFxv_2, an O-antigen PEtN modification plasmid in Shigella flexneri. Glycobiology 2014 Mar;24(3):305-313.
- 37. Jakhetia R, Talukder KA, Verma NK. Isolation, characterization and comparative genomics of bacteriophage SfIV: a novel serotype converting phage from Shigella flexneri. BMC Genomics 2013 Oct;14(1):677.
- 38. Zhang N, Lan R, Sun Q, Wang J, Wang Y, Zhang J, et al. Genomic portrait of the evolution and epidemic spread of a recently emerged multidrug-resistant Shigella flexneri clone in China. J Clin Microbiol 2014 Apr;52(4):1119-1126.
- 39. Chen J-H, Hsu W-B, Chiou C-S, Chen C-M. Conversion of Shigella flexneri serotype 2a to serotype Y in a shigellosis patient due to a single amino acid substitution in the protein product of the bacterial glucosyltransferase gtrII gene. FEMS Microbiol Lett 2003 Jul;224(2):277-283.
- 40. Sun Q, Lan R, Wang Y, Wang J, Luo X, Zhang S, et al. Genesis of a novel Shigella flexneri serotype by sequential infection of serotype-converting bacteriophages SfX and SfI. BMC Microbiol 2011 Dec;11(1):269.
- 41. Qiu S, Wang Z, Chen C, Liu N, Jia L, Liu W, et al. Emergence of a novel Shigella flexneri serotype 4s strain that evolved from a serotype X variant in China. J Clin Microbiol 2011 Mar;49(3):1148-1150.
- 42. Li S, Sun Q, Wei X, Klena JD, Wang J, Liu Y, et al. Genetic characterization of Shigella flexneri isolates in Guizhou Province, China. PLoS One 2015 Jan;10(1):e0116708.
- 43. Parajuli P, Deimel LP, Verma NK. Genome analysis of Shigella flexneri serotype 3b strain SFL1520 reveals significant horizontal gene acquisitions including a multidrug resistance cassette. Genome Biology and Evolution 2019 Mar;11(3):776-785.
- 44. Parajuli P, Adamski M, Verma NK. Bacteriophages are the major drivers of Shigella flexneri serotype 1c genome plasticity: a complete genome analysis. BMC Genomics 2017 Sep;18(1):722.
- 45. Nie H, Yang F, Zhang X, Yang J, Chen L, Wang J, et al. Complete genome sequence of Shigella flexneri 5b and comparison with Shigella flexneri 2a. BMC Genomics 2006 Jul;7(1):173.
- 46. Karimi-Yazdi M, Ghalavand Z, Shabani M, Houri H, Sadredinamin M, Taheri M, et al. High rates of antimicrobial resistance and virulence gene distribution among Shigella spp. isolated from pediatric patients in Tehran, Iran. Infect Drug Resist 2020 Feb;13:485-492.
- 47. West NP, Sansonetti P, Mounier J, Exley RM, Parsot C, Guadagnini S, et al. Optimization of virulence functions through glucosylation of Shigella LPS. Science 2005 Feb;307(5713):1313-1317.
- 48. Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 2003 Dec;67(4):593-656.
- 49. Morona R, Daniels C, Van Den Bosch L. Genetic modulation of Shigella flexneri 2a lipopolysaccharide O antigen modal chain length reveals that it has been optimized for virulence. Microbiology (Reading) 2003 Apr;149(Pt 4):925-939.
- 50. Hong M, Payne SM. Effect of mutations in Shigella flexneri chromosomal and plasmid-encoded lipopolysaccharide genes on invasion and serum resistance. Mol Microbiol 1997 May;24(4):779-791.
- 51. Carter JA, Blondel CJ, Zaldívar M, Álvarez SA, Marolda CL, Valvano MA, et al. O-antigen modal chain length in Shigella flexneri 2a is growth-regulated through RfaH-mediated transcriptional control of the wzy gene. Microbiology (Reading) 2007 Oct;153(Pt 10):3499-3507.
- 52. Watson JL, Sanchez-Garrido J, Goddard PJ, Torraca V, Mostowy S, Shenoy AR, et al. Shigella sonnei O-antigen inhibits internalization, vacuole escape, and inflammasome activation. mBio 2019 Dec;10(6):799379.
- 53. Brengi SP, Sun Q, Bolaños H, Duarte F, Jenkins C, Pichel M, et al. PCR-based method for shigella flexneri serotyping: international multicenter validation. J Clin Microbiol 2019 Mar;57(4):e01592-18.
- 54. Coimbra RS, Grimont F, Grimont PA. Identification of Shigella serotypes by restriction of amplified O-antigen gene cluster. Res Microbiol 1999 Oct;150(8):543-553.
- 55. Li Y, Cao B, Liu B, Liu D, Gao Q, Peng X, et al. Molecular detection of all 34 distinct O-antigen forms of Shigella. J Med Microbiol 2009 Jan;58(Pt 1):69-81.
- 56. Sun Q, Lan R, Wang Y, Zhao A, Zhang S, Wang J, et al. Development of a multiplex PCR assay targeting O-antigen modification genes for molecular serotyping of Shigella flexneri. J Clin Microbiol 2011 Nov;49(11):3766-3770.
- 57. Wu Y, Lau HK, Lee T, Lau DK, Payne J. In silico serotyping based on whole-genome sequencing improves the accuracy of Shigella identification. Applied and Environmental Microbiology 2019 Apr 1;85(7):e00165-19.
- 58. Zhao L, Xiong Y, Meng D, Guo J, Li Y, Liang L, et al. An 11-year study of shigellosis and Shigella species in Taiyuan, China: active surveillance, epidemic characteristics, and molecular serotyping. J Infect Public Health 2017 Nov - Dec;10(6):794-798.
- 59. Gentle A, Ashton PM, Dallman TJ, Jenkins C. Evaluation of molecular methods for serotyping Shigella flexneri. J Clin Microbiol 2016 Jun;54(6):1456-1461.