COVID-19 initially emerged in China towards the end of 2019 and spread all over the globe in a very short time.1 The clinical manifestations of COVID-19 vary from asymptomatic to severe respiratory failure requiring assisted ventilation and oxygenation.2 Little is known about the effects of COVID-19 disease in cystic fibrosis (CF) cases.3 Although CF patients always had a higher risk of complications from respiratory viral infections, not much is known if this is also applicable to COVID-19 disease.4
Case Report
A 14-year-old-female, was a known CF patient with restrictive lung disease, exocrine pancreatic insufficiency, and failure to thrive. She had a homozygous variant (c.1069G>A, p.Ala357Thr(A357T)) of CF, and her respiratory cultures chronically grew pseudomonas. She presented to the emergency department of our tertiary care hospital with a four-day history of an increasing lethargy and fatigue with an increased chronic productive cough associated with occasional yellowish sputum. Undocumented fever and diminished oral intake were also reported.
Examination revealed her to be hypoxemic with oxygen saturation of 60% (via pulse oximeter) in the room air that picked up to 94% with 15 L of oxygen via non-rebreathing mask. Her respiratory rate was 32 breaths per minute and the blood pressure was 90/52 mmHg. As she was dehydrated, intravenous rehydration therapy was initiated.
Her nasopharyngeal reverse transcriptase chain reaction test came positive for COVID-19.
She was directly admitted to the COVID-19 isolation ward and commenced on parenteral broad-spectrum antibiotics (piperacillin-tazobactam) based on known sensitivity from her previous sputum cultures, dexamethasone (6 mg intravenously as part of COVID-19 local management policy), and pharmacological deep venous thrombosis prophylaxis (enoxaparin 4000 IU subcutaneously once daily).
The patient’s clinical condition significantly improved in three days and she had no more increased cough, no fever, no significant shortness of breath, and was able to maintain saturation of ≥ 95% in ambient air. She was back to her baseline, with no known major sequelae.
The patient’s laboratory investigation details on admission and discharge are listed in Table 1. The chest X-ray at presentation is shown in Figure 1.
Table 1: Laboratory investigation results at admission and discharge.
|
Hemoglobin
|
9.7 g/dL
|
9.1 g/dL
|
11–14.5 g/dL
|
|
White cell count
|
17.7 × 109/L
|
7.2 × 109/L
|
2.4–9.5 × 109/L
|
|
Neutrophils
|
14.2 × 109/L
|
5.9 × 109/L
|
1–4.8 × 109/L
|
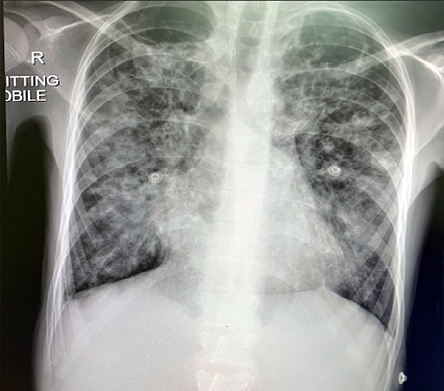 Figure 1: Chest X-ray on admission showing bilateral infiltrates involving more than 50% of the lung zones.
Figure 1: Chest X-ray on admission showing bilateral infiltrates involving more than 50% of the lung zones.
Discussion
CF is an autosomal recessive inherited disease that is estimated to affect 80 000 people worldwide. It is caused by a mutation in the CF transmembrane regulatory gene.5 To date, out of the nearly 2000 CF transmembrane regulatory mutations identified, around 300 are known to cause CF.6 Although the disease affects multi organs including the liver, intestine, and pancreas, most of its morbidity and mortality are pulmonary in nature due to repeated respiratory inflammation and infection.7 The limited evidence available to date indicates lower incidence rates of COVID-19 among CF patients, with no significant correlation between the two diseases.8 At the initial stages of the pandemic, most specialists thought otherwise—that this category of patients would have worse prognosis.3 However, the CF registries from eight European countries that described 40 CF patients with confirmed COVID-19 disease have not shown any unique risk factor for acquiring or rendering the illness more severe among this heterogeneous group.9 Another study from Spain showed CF patients having lower mortality from COVID-19 compared to the general population.10 However, being a juncture of the global health crisis with shortages in ventilators and lifesaving resources, several health institutes had made policies limiting their support to CF patients and others with chronic disabilities presuming that they might not have good prognosis if infected with COVID-19.11
Studies from different countries showed a lower incidence of COVID-19 in CF patients (0.14%) in comparison to the general population (0.58%).12 CF patients’ habitually strict adherence to hand hygiene measures, masking, and social distancing may have helped reduce their exposure to the virus.11,12
The clinical manifestations of CF exacerbation attacks might overlap with symptoms of active COVID-19 disease like cough, fever, increasing shortness of breath, and change in the color or the consistency of the expectorated sputum, as in the current case. Differentiating CF exacerbation from COVID-19 disease may not always be straightforward and this reveals the importance of maintaining a low threshold for screening CF patients for COVID-19.4
Although our patient presented with severe COVID-19 disease (high respiratory rate > 30 per minute, low oxygen saturation (60%), and bilateral infiltrates in > 50% of the lung fields),13 she responded very well to the antibiotic and dexamethasone treatment, and after three days she was able to maintain satisfactory oxygen saturation in room air. She was discharged to continue home quarantine and she was back to her baseline.
The full mechanism of mild COVID-19 disease course in general in CF patients is not fully understood. It is thought that the disruption of interleukin-6 signaling in CF lungs that occurs through increased serine protease release and subsequent cleavage of both membrane-bound and soluble interleukin-6 receptors may play an important role in the relatively milder manifestation of COVID-19 in CF patients. Moreover, the fact that most CF patients are on regular immunomodulatory medications (e.g., azithromycin) and DNase (mucolytic) might also contribute to this relative protection.14
Conclusion
The present case, supported by reports from elsewhere, suggests that CF patients are likely to have a lower incidence and milder course of COVID-19 than the general population. The mechanism responsible for this phenomenon is still quite unclear and warrants closer evaluation. Policies need to be modified to prevent discrimination against providing treatment and life-saving measures for CF patients during pandemics.
Disclosure
The authors declared no conflicts of interests. A consent was provided.
references
- 1. Al Lawati A, Khamis F, Al Habsi S, Al Dalhami K. Risk of COVID-19 infection in healthcare workers exposed during use of non-invasive ventilation in a tertiary care hospital in Oman. Oman Med J 2021 Mar;36(2):e236.
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020;323(13):1239-1242.
- 3. Mirza AA, Rad EJ, Mohabir PK. Cystic fibrosis and COVID-19: care considerations. Respir Med Case Rep 2020;31:101226.
- 4. Colombo C, Burgel PR, Gartner S, van Koningsbruggen-Rietschel S, Naehrlich L, Sermet-Gaudelus I, et al. Impact of COVID-19 on people with cystic fibrosis. Lancet Respir Med 2020 05;8(5):e35-e36.
- 5. Davies JC, Alton EW, Bush A. Cystic fibrosis. BMJ 2007 Dec;335(7632):1255-1259.
- 6. Fainardi V, Longo F, Chetta A, Esposito S, Pisi G. Sars-CoV-2 infection in patients with cystic fibrosis. An overview. Acta Bio Medica: Atenei Parmensis 2020;91(3):e2020035.
- 7. Corvol H, de Miranda S, Lemonnier L, Kemgang A, Reynaud Gaubert M, Chiron R, et al. First wave of COVID-19 in French patients with cystic fibrosis. J Clin Med 2020 Nov;9(11):E3624.
- 8. Mathew HR, Choi MY, Parkins MD, Fritzler MJ. Systematic review: cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic. BMC Pulm Med 2021 May;21(1):173.
- 9. Cosgriff R, Ahern S, Bell SC, Brownlee K, Burgel PR, Byrnes C, et al. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cyst Fibros 2020 05;19(3):355-358.
- 10. Mondejar-Lopez P, Quintana-Gallego E, Giron-Moreno RM, Cortell-Aznar I, Ruiz de Valbuena-Maiz M, Diab-Caceres L, et al; CF-COVID19-Spain Registry Group. Impact of SARS-CoV-2 infection in patients with cystic fibrosis in Spain: Incidence and results of the national CF-COVID19-Spain survey. Respir Med 2020 Aug - Sep;170:106062.
- 11. Ladores S. The unique challenges and lessons imparted by the cystic fibrosis community in the time of COVID-19 pandemic. J Patient Exp 2020 Aug;7(4):442-443.
- 12. Chapman KD, Moffett KS. Cystic fibrosis and COVID-19. South Med J 2020 09;113(9):422.
- 13. Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med 2020 Dec;383(25):2451-2460.
- 14. Bezzerri V, Lucca F, Volpi S, Cipolli M. Does cystic fibrosis constitute an advantage in COVID-19 infection? Ital J Pediatr 2020 Oct;46(1):143.