Despite guidelines recommending no need for coagulation profile testing before ear, nose, and throat (ENT) surgeries when proper bleeding history is taken (detailed history of previous surgery and trauma, a family history, and detail of anti-thrombotic medication),1 local surgical practices have not changed. The high percentage of abnormal laboratory results not associated with significant bleeding disorders leads to over investigation and unnecessary concerns by surgeons.2 Cost and delaying surgeries are major issues faced when insignificant abnormalities are found in the coagulation profile results.3 In 2008, the British Committee for Standards of Hematology published guidelines on assessing bleeding before surgeries or invasive procedures. It stated that the indication for sending a coagulation testing (prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time, fibrinogen level, and platelet count) is based on the bleeding history of the patient. Bleeding history includes personal or family history of bleeding following trauma or surgery and details of any anti-thrombotic medication. It states clearly that patients with negative bleeding history do not require coagulation testing.1 Also, the risk of postoperative bleeding depends on the extent of tissue perfusion, the condition of the wound, and the type of surgery.4 Moreover, low and moderate-risk bleed surgeries do not need prior coagulation profile testing when the history of bleeding challenge is negative.4,5 Many surgeons in our region still send coagulation profile for every single patient undergoing ENT surgeries regardless of the risk of bleeding or negative history of bleeding challenge. This study aimed to measure an objective estimate of hemostatic outcomes in ENT surgeries concerning coagulation testing as a primary outcome. In addition, we evaluate the reasons ENT surgeons perform preoperative coagulation testing as a secondary objective.
Methods
This multi-center retrospective cohort study was conducted in three tertiary hospitals located in Muscat, the capital of Oman, including Sultan Qaboos University Hospital, Armed Force Hospital, and Al-Nahdha Hospital. All patients who underwent ENT surgeries from 1 July 2017 to 1 January 2018 were enrolled in the study. The study was approved by the Sultan Qaboos University ethics committee. All participants provided informed consent/assent before proceeding with the study.
All pediatrics and adult patients who had ENT surgeries in the three main tertiary hospitals were included in the study. The retrieved data included gender, age, type of surgery, results of coagulation blood test (if done), other laboratory test results (complete blood count, biochemical profile, etc.), postoperative bleeds, how it was managed, need for blood transfusion, and whether the patient required another surgery to stop the bleeding. Patients with known bleeding history, previous coagulation derangement, taking anticoagulants, pregnant, acute renal failure, sepsis, and known chronic medical conditions (cancer, collagenic disorders, chronic liver disease, and chronic renal failure) were excluded from the study.
During the study period, 730 patients were included in the study. Ages ranged from one month to 85 years. Patients were divided into two groups according to whether coagulation profile testing was performed. Three hundred and seventy-two patients were asked a detailed personal and family history, which included bleeding history (due to trauma/surgery). The other 358 patients underwent a detailed history and preoperative coagulation testing, which included PT, aPTT, and fibrinogen levels. Patients were subcategorized according to the surgery they underwent into major or minor surgeries. Major surgeries included basal skull surgery, adenotonsillectomy, thyroidectomy, drainage of effusion in otitis media, and peritonsillar abscess drainage. Minor surgeries included foreign body removal, functional endoscopic sinus surgery, and other endoscopies. We divided postoperative bleed rates in patients into primary (within 48 hours) and secondary bleeds (up to 10 days), and recorded how the bleeding was managed and if they required any blood products support.
At the same time, all ENT surgeons (n = 57) from the three hospitals were interviewed. They were asked about their decision to take a preoperative history alone or do coagulation testing, and their reason for ordering the coagulation profile. They were offered the choice to give more than one reason for sending pre-surgical coagulation testing.
Quantitative variables were expressed as mean and standard deviation (SD). They were compared by chi-square test or analysis of variance whenever appropriate. A p-value ≤ 0.050 was considered statistically significant. Statistical analyses were performed using SPSS (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) and GraphPad Prism-5.0 (GraphPad Software).
Results
The study included data from 730 patients (432 males and 298 females) who underwent ENT surgical procedures. Their mean age was 19.6±16.9 years. Out of the 730 patients, 372 patients were interviewed for bleeding history (group 1), and 358 had a preoperative coagulation profile check (group 2) [Table 1]. There was no statistically significant difference between the two groups regarding gender distribution (chi-square = 0.651, p-value < 0.419). However, regarding the age distribution of the two groups, there was a highly significant difference as most of the older age group (≥ 40) had coagulation testing (chi-square = 37.964, p < 0.001).
Fourteen patients (1.9%) developed postoperative bleeding [Table 2]. Two patients had a minor intraoperative bleed that required stitching (one in each group). Two patients in group 2 had postoperative secondary bleeding after one week that responded to local hemostatic measures. None was due to abnormal bleeding tendency, and they did not require any hemostatic support. Six bled early (primary hemorrhage) while at the hospital due to surgical reasons (surgical site bleed that required suturing). Eight patients had delayed postoperative bleeds after being discharged (due to eating hard food/trauma). Four patients had major bleeds requiring surgical intervention. Coagulation profile testing was repeated in 55.0% of tested patients, and more than half had coagulation and Von Willebrand factor (VWF) assays. The time wasted for patients waiting to be cleared for surgery ranged between six to 14 months (median = 9 months).
Around half of surgeons (50.9%) do not do coagulation testing and depend solely on history of bleeding challenge. Twenty-eight surgeons who preferred to do the coagulation profile for their patients answered the survey. Twenty-two (78.6%) gave the reason for a habitual practice and overprotection. Two were not confident with the current evidence, two had previous serious bleeding experiences with their patients, one was not confident with the history, and one feared medico-legal issues [Figure 1].
Table 1: Demographic and laboratory data of patients undergoing ENT surgeries in both groups.
|
Gender |
|
|
|
|
|
Male |
226 (60.8%) |
206 (57.5%) |
432 (59.2%) |
0.419 |
|
Female |
146 (39.2%) |
152 (42.5%) |
298 (40.8%) |
|
|
Age group, years |
|
|
|
< 0.001 |
|
< 20 |
250 (67.2%) |
176 (49.2%) |
426 (58.4%) |
|
|
20–40 |
101 (27.2%) |
116 (32.4%) |
217 (29.7%) |
|
|
40–60 |
15 (4.0%) |
55 (15.4%) |
70 (9.6%) |
|
|
> 60 |
6 (1.6%) |
11 (3.1%) |
17 (2.3%) |
|
|
Laboratory testing |
|
|
|
|
|
Repeat coagulation profile (twice or more) |
- |
197 (55.0%) |
|
|
|
Coagulation factor assay |
- |
106 (29.6%) |
|
|
|
VWF and coagulation factor assay |
- |
77 (21.5%) |
|
|
|
Time wasted before surgery, months |
|
|
|
|
VWF: Von Willebrand factor.
Table 2: Surgical procedures and postoperative bleeding events in both groups.
|
Postoperative bleeding |
|
Primary |
3 (0.8%) |
3 (0.8%) |
0.580 |
|
Secondary |
6 (1.6%) |
2 (0.6%) |
|
|
Control of bleeding |
|
Spontaneous/bedside |
4 (1.1%) |
2 (0.6%) |
1.000 |
|
Operating theatre |
5 (1.3%) |
3 (0.8%) |
|
|
Surgical procedure |
|
Major |
370 (99.5%) |
354 (89.9%) |
0.440 |
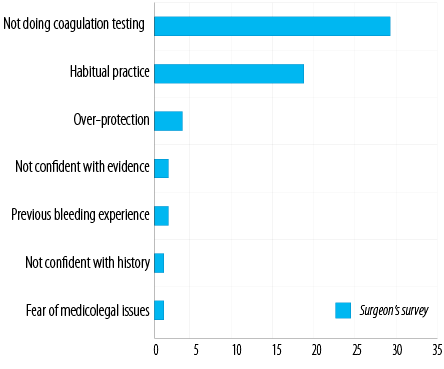
Figure 1: Number of ear, nose, and throat surgeons not doing coagulation testing (n = 29) and reasons given for performing coagulation testing (n = 28).
Discussion
We found that preoperative testing is overused and coagulation profile testing for patients before low or moderate bleeding risk ENT surgeries is still widely practiced. Almost 51.0% of patients had coagulation profile testing despite a negative history of bleeding challenges. Moreover, in 55.0%, the test was repeated once or more, along with coagulation factor and Von Willebrand disease (VWD) assays. We also found that neither coagulation testing nor abnormal results were associated with postoperative bleeding outcomes.
Normal coagulation profile results do not reliably exclude all bleeding disorders. Of note, platelet function disorders, factor XIII deficiency, VWD type 2N, and vessel wall abnormalities are associated with perfectly normal coagulation screen results.6–10 On the other hand, an abnormal coagulation profile does not necessarily indicate an underlying factor deficiency.11,12 Pre-analytical errors, prekallikrein deficiency, high-molecular-weight kininogen, lupus anticoagulant, and other circulating inhibitors give rise to abnormal coagulation profiles and are not associated with any significantly increased bleeding risk.13–16 Moreover, factor XII deficiency is often encountered in this part of the world due to consanguineous marriages.17 Despite an alarmingly prolonged aPPT associated with the disorder, it is well known that it does not increase the risk of bleeding.18 The fact that 55.0% of the coagulation testing in our set-up was abnormal and repeated highlights the major burden of re-testing and performing unnecessary coagulation and VWF assays, which aggravates the patient and family anxiety and suffering, results in unnecessary delays, cancellations, and rescheduling of surgeries.
A review of the guidelines for bleeding risk does not support coagulation testing before procedures for assessing bleeding risk and emphasized that abnormal coagulation results could not predict the increased risk of operative hemorrhage or the risk of bleeding postoperatively.19
Our data support that coagulation testing had no added value in predicting postoperative bleeding risk. Many surgeons send the preoperative tests out of habit and not evidence-based. Surgeons need to be educated more on the current evidence of the superiority of the history of bleeding challenge over the routine coagulation tests. Preoperative testing should be done only if patient history suggests an underlying bleeding tendency.
Our study is limited by its retrospective nature and missing some initial and follow-up laboratory data.
Conclusion
Despite the guidelines recommending no coagulation testing before surgeries, many local ENT surgeons still consider them a standard practice to evaluate the patients bleeding risk prior to any surgical procedure. This practice resulted in unnecessary delays in surgeries (reaching more than a year in many patients) in addition to increased parent/patient anxiety and increased total cost. Therefore, we recommend adhering to guidelines of taking a detailed hemostatic history. We also recommend using standardized questionnaires; for example, the Condensed Molecular and Clinical Markers for the Diagnosis and Management of Type 1 questionnaire.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Chee YL, Crawford JC, Watson HG, Greaves M; British Committee for Standards in Haematology. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Br J Haematol 2008 Mar;140(5):496-504.
- 2. Burk CD, Miller L, Handler SD, Cohen AR. Preoperative history and coagulation screening in children undergoing tonsillectomy. Pediatrics 1992 Apr;89(4 Pt 2):691-695.
- 3. Bhasin N, Parker RI. Diagnostic outcome of preoperative coagulation testing in children. Pediatr Hematol Oncol 2014 Aug;31(5):458-466.
- 4. Thiele T, Kaftan H, Hosemann W, Greinacher A. Hemostatic management of patients undergoing ear-nose-throat surgery. GMS Curr Top Otorhinolaryngol Head Neck Surg 2015 Dec;14:Doc07.
- 5. Thiruvenkatarajan V, Pruett A, Adhikary SD. Coagulation testing in the perioperative period. Indian J Anaesth 2014 Sep;58(5):565-572.
- 6. Shapiro AD. Platelet function disorders. Haemophilia 2000 Jul;6(Suppl 1):120-127.
- 7. Karimi M, Peyvandi F, Naderi M, Shapiro A. Factor XIII deficiency diagnosis: challenges and tools. Int J Lab Hematol 2018 Feb;40(1):3-11.
- 8. Casonato A, Galletta E, Sarolo L, Daidone V. Type 2N von Willebrand disease: characterization and diagnostic difficulties. Haemophilia 2018 Jan;24(1):134-140.
- 9. Miesbach W, Berntorp E. Von Willebrand disease - the ‘Dos’ and ‘Don’ts’ in surgery. Eur J Haematol 2017 Feb;98(2):121-127.
- 10. De Paepe A, Malfait F. Bleeding and bruising in patients with Ehlers-Danlos syndrome and other collagen vascular disorders. Br J Haematol 2004 Dec;127(5):491-500.
- 11. Chng WJ, Sum C, Kuperan P. Causes of isolated prolonged activated partial thromboplastin time in an acute care general hospital. Singapore Med J 2005 Sep;46(9):450-456.
- 12. Nizamoglu M, Alexander KS, Anwar U, Bhandari S. Isolated APTT prolongation-not always a bleeding risk in acute paediatric burns surgery. Eur J Plast Surg 2014;37(12):695-696.
- 13. Lippi G, Favaloro EJ. Preanalytical issues in hemostasis and thrombosis testing. Methods Mol Biol 2017;1646:29-42.
- 14. Zheng S, Just S, Brighton T. Prekallikrein deficiency. Pathology 2016 Oct;48(6):634-637.
- 15. Malbora B, Bilaloglu E. Lupus anticoagulant positivity in pediatric patients with prolonged activated partial thromboplastin time: a single-center experience and review of literature. Pediatr Hematol Oncol 2015;32(7):495-504.
- 16. Duran-Suarez JR. Incidence of circulating anticoagulants in a normal population. Acta Haematol 1982;67(3):217-219.
- 17. Al-Fawaz IM, Gader AM, Bahakim HM, Al-Mohareb F, Al-Momen AK, Harakati MS. Hereditary bleeding disorders in Riyadh, Saudi Arabia. Ann Saudi Med 1996 May;16(3):257-261.
- 18. Stavrou E, Schmaier AH. Factor XII: what does it contribute to our understanding of the physiology and pathophysiology of hemostasis & thrombosis. Thromb Res 2010 Mar;125(3):210-215.
- 19. van Veen JJ, Spahn DR, Makris M. Routine preoperative coagulation tests: an outdated practice? Br J Anaesth 2011 Jan;106(1):1-3.