Coronaviruses are a family of single-stranded RNA viruses causing infections in animals and humans.1 COVID-19 is caused by novel coronavirus SARS-CoV-2. Since the onset of the COVID-19 pandemic, a wide spectrum of gastrointestinal complications and few cases of acute pancreatitis have been reported in association with COVID-19 infection.1 Here, we report a unique case of acute necrotizing pancreatitis caused by COVID-19 with hyperglycemia and normal amylase and lipase levels.
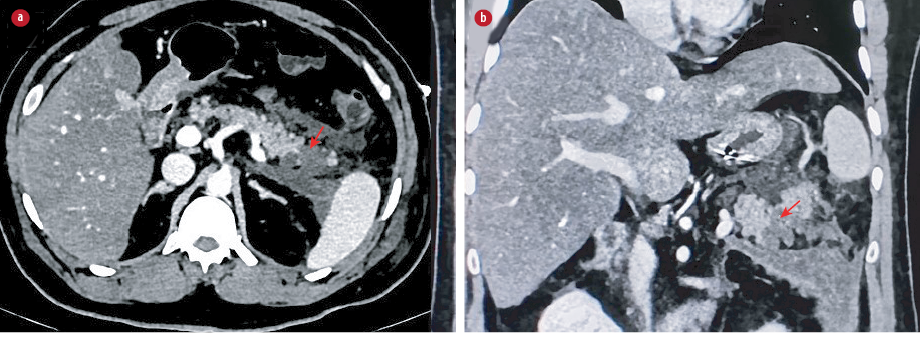
Figure 1: CECT abdomen axial and coronal sections shows non-enhancing hypo-attenuating necrotic collection involving the tail of the pancreas (red arrows) with extension into left anterior peripancreatic and
pararenal spaces.

Figure 2: High resolution computed tomography chest lung window, axial and coronal sections (a and b) show multiple confluent and discrete, mixed subpleural and peribronchovascular consolidations with ground-glass opacities and interstitial septal thickening involving both lungs. HRCT chest mediastinal window, axial section (c) shows bilateral pleural effusion.
Case report
A 35-year-old male presented to the emergency department with complaints of abdominal pain, fever, and mild cough lasting for five days. He was not an alcoholic and had no history of trauma, chronic disease, or medication use. On examination, he was febrile with tenderness in the epigastric region, and oxygen saturation was maintained. Laboratory investigations revealed normal serum amylase (46 U/L; units per liter) and lipase levels (42 U/L). His gamma-glutamyl transferase levels were elevated (149 U/L), and liver function tests were normal. Random blood glucose was elevated (240 mg/dL), and serum calcium (9 mg/dL) and lipid profile (triglycerides: 130 mg/dL) were normal. The patient was subjected to abdominal contrast-enhanced computed tomography (CECT), which showed non-enhancing necrotic areas involving the tail of the pancreas with fluid and fat stranding in the peripancreatic spaces and thickening of the adjacent fascial planes. The rest of the pancreas was unremarkable. Bilateral minimal pleural effusion was noted. A diagnosis of acute necrotizing pancreatitis was given on imaging (modified CT severity index: 8/10) [Figure 1]. No cholelithiasis or choledocholithiasis was seen on CT. The visualized base of lungs showed ground-glass opacities and interstitial septal thickening. The patient underwent a dedicated chest CT, which revealed bilateral confluent and discrete, subpleural and peribronchovascular consolidations with ground glass opacities [Figure 2]. Given the typical imaging findings for COVID-19, the patient underwent reverse transcription polymerase chain reaction (RT-PCR) for COVID-19, which was positive. Initially, he was treated conservatively with bowel rest, analgesics, intravenous fluid resuscitation, and empirical antibiotics. Serum amylase and lipase levels were repeated the next day and remained within normal limits. The patient’s symptoms gradually improved, and he was discharged.
Discussion
Acute pancreatitis is caused by various etiologies, including gallstones, alcohol abuse, hypertriglyceridemia, hypercalcemia, iatrogenic causes, infections, autoimmune and hereditary conditions, chronic medications, and trauma.2 A wide range of bacterial, viral, fungal, and parasitic organisms can cause pancreatitis. Mumps, coxsackie, measles, and Hepatitis viruses have been implicated in causing pancreatitis.3 The diagnosis of acute pancreatitis requires any two of the following three criteria: acute onset of severe upper abdominal pain, consistent with pancreatitis; elevation of serum amylase or lipase, three times the upper limit of normal; and characteristic imaging findings.4 CECT abdomen has very high sensitivity and specificity of 90% for diagnosis of pancreatitis.5 Revised Atlanta classification categorizes acute pancreatitis into interstitial edematous and necrotizing pancreatitis based on imaging findings. Acute necrotizing pancreatitis typically occurs by 48–72 hours after the disease onset.6 Our patient had typical abdominal pain, and imaging showed pancreatic necrosis with peripancreatic inflammation and collection leading us to the diagnosis of acute necrotizing pancreatitis. A study states that CT is less sensitive in picking the necrosis before four days of onset.7 CECT is generally recommended after 4–5 days of onset when the necrosis starts to appear radiologically.8 Our case had mild non-enhancing necrotic areas only in the tail region. So, we presumed that CT was showing an early phase of necrosis. Chest CT revealed ground-glass opacities and consolidation, which were proven to be secondary to COVID-19 by RT-PCR. Our patient had no history of alcohol intake, trauma, medications, or any prior interventions, with normal triglyceride and calcium values and CT of the abdomen showing no evidence of biliary stones. Since CT chest findings and RT-PCR confirmed COVID-19, we ascertain that acute necrotizing pancreatitis was also caused by COVID-19 infection.
COVID-19 pancreatitis is thought to be mediated by angiotensin-converting enzyme-2 (ACE-2) receptors on the host cells, which are highly expressed in the pancreatic cells.9 Possible pathogenesis include the direct cytopathic effect of local viral replication or by the immune response induced by the virus indirectly. Our case had a unique finding that serum amylase and lipase levels were not elevated, both at admission and the next day. Serum amylase has low specificity and can be elevated in various conditions like cholecystitis and also be normal in alcohol and hypertriglyceridemia induced pancreatitis.10 Lipase is produced and stored in pancreatic acinar cells; hence, it is very specific and has a negative predictive value of 94–100% in diagnosing pancreatitis.11 The literature shows that lipase levels increase within 4–8 hours of acute pancreatitis onset to peak at 24 hours. Amylase levels rise after 6–24 hours and peak at 48 hours. These enzymes stay elevated in the bloodstream for about 7–14 days. However, it was peculiar to note the normal enzyme levels despite the ongoing infective and inflammatory processes in our case.12 Many isolated case reports have documented that acute pancreatitis can have normal lipase levels.4,5,10,11 In acute or chronic pancreatitis cases, both amylase and lipase levels may remain normal due to loss of secretory function after chronic inflammation and fibrosis of the gland. In the case series of COVID-19 associated pancreatitis published by Wang et al,13 three patients had elevated amylase but normal lipase levels. To the best of our knowledge, this is the first case of COVID-19 pancreatitis with normal lipase and amylase levels.
Another finding in our case was hyperglycemia. Hyperglycemia in a COVID-19 patient with pancreatitis could be due to the direct cytopathic effect of the virus, as the islet cells are more concentrated in the pancreatic tail region and show high expression of the ACE-2 receptors.14 Other possible reasons for hyperglycemia include stress-induced transient hyperglycemia and incidentally detected pre-existing diabetes.15 Diabetes workup including glycated hemoglobin assay may be useful in ascertaining the cause of hyperglycemia. Identifying the COVID-19 patients with pancreatitis is of paramount importance as they usually have a severe illness on admission and multisystem involvement with an accelerated clinical course. Including the upper abdomen in CT chest plain study for COVID-19 pneumonia also helps in the early identification of pancreatitis. CECT plays an important role in confirming the diagnosis and identification of necrosis, assessing severity, and initiating appropriate treatment.
Conclusion
COVID-19 patients can present as acute pancreatitis with normal serum amylase and lipase levels. Emergency physicians should be aware of this diagnostic conundrum, have a high index of suspicion in patients presenting with acute abdominal pain during this pandemic, and set a low threshold for further evaluation with imaging.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Carroll J, Herrick B, Gipson T, Lee S. Acute pancreatitis: diagnosis, prognosis and treatment. Am Fam Physician 2007;75(10):1513-1520.
- 2. Chatila AT, Bilal M, Guturu P. Evaluation and management of acute pancreatitis. World J Clin Cases 2019 May;7(9):1006-1020.
- 3. Rawla P, Bandaru SS, Vellipuram AR. Review of infectious etiology of acute pancreatitis. Gastroenterology Res 2017 Jun;10(3):153-158.
- 4. Singh A, Shrestha M, Anand C. Acute pancreatitis with normal amylase and lipase–an ED dilemma. Am J Emerg Med 2016 May;34(5):940.e5-940.e7.
- 5. Cartier T, Sogni P, Perruche F, Meyniard O, Claessens YE, Dhainaut JF, et al. Normal lipase serum level in acute pancreatitis: a case report. Emerg Med J 2006 Sep;23(9):701-702.
- 6. Shyu JY, Sainani NI, Sahni VA, Chick JF, Chauhan NR, Conwell DL, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014 Sep-Oct;34(5):1218-1239.
- 7. Spanier BW, Nio Y, van der Hulst RW, Tuynman HA, Dijkgraaf MG, Bruno MJ. Practice and yield of early CT scan in acute pancreatitis: a Dutch observational multicenter study. Pancreatology 2010;10(2-3):222-228.
- 8. Rashid MU, Hussain I, Jehanzeb S, Ullah W, Ali S, Jain AG, et al. Pancreatic necrosis: complications and changing trend of treatment. World J Gastrointest Surg 2019 Apr;11(4):198-217.
- 9. Kataria S, Sharif A, Ur Rehman A, Ahmed Z, Hanan A. COVID-19 induced acute pancreatitis: a case report and literature review. Cureus 2020 Jul;12(7):e9169.
- 10. Mathur AK, Whitaker A, Kolli H, Nguyen T. Acute pancreatitis with normal serum lipase and amylase: a rare presentation. J Pancreas (Online) 2016 Jan;17(1):98-101.
- 11. Shah AM, Eddi R, Kothari ST, Maksoud C, DiGiacomo WS, Baddoura W. Acute pancreatitis with normal serum lipase: a case series. JOP 2010 Jul;11(4):369-372.
- 12. Jasdanwala S, Babyatsky M. A critical evaluation of serum lipase and amylase as diagnostic tests for acute pancreatitis. Integr Mol Med 2015;2(3):189-95.
- 13. Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology 2020 Jul;159(1):367-370.
- 14. Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, et al. Regional differences in islet distribution in the human pancreas–preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One 2013 Jun;8(6):e67454.
- 15. Thaweerat W. Current evidence on pancreatic involvement in SARS-CoV-2 infection. Pancreatology 2020 Jul;20(5):1013-1014.