Endometrial carcinomas (EC) are among the most common malignancies involving the female genital tract and contribute to significant morbidity and mortality worldwide.1 Clinico-pathological, molecular, and epidemiological studies established the occurrence of two major types of EC, namely type I and type II (EC-I and EC-II, respectively).2
EC-I is the most common type of EC, for which the histologic model is endometrial endometrioid carcinoma (EEC). EEC represents the most frequent type (80% of cases).3
EC-II is considerably less widespread than EC-I (15% of cases) and is represented by endometrial serous carcinoma (ESC) and endometrial clear cell carcinoma (ECCC). Many genetic abnormalities are detected in EC-I, including phosphatase and tensin homolog (PTEN) inactivation, beta-catenin (CTNNB1) gene mutations, microsatellite instability, and activating mutations of the Kras gene.
EC-I frequently express hormone receptors and have a strong association with unopposed estrogen stimulation. On the other hand, EC-II exhibits a low rate of expression of hormonal receptors and is not significantly associated with clinical conditions linked to excessive estrogen production. EC-II, namely ESC, disclose common mutations and overexpression of the p53 gene, and less frequently, HER2/neu genes.3
Clinically, EC are graded according to the International Federation of Gynecology and Obstetrics (FIGO) system,4 which considers microscopic morphological parameters such as nuclear size and shape, glandular formation, mitoses, and solid tumor components. Classically, they have been divided into two types: the more common estrogen-dependent EC-I and the less common, yet more dreadful, estrogen-independent EC-II.2,3
Although EC-II is classified as estrogen-independent, new data propose that both EC-I and EC-II share several general risk factors, including age at menarche, parity, and contraceptive use, indicating that steroids have an impact on the risk of developing either type.5
Steroid hormones receptor, namely estrogen receptor (ER) and progesterone receptor (PR) expression, along with their etiological and prognostic roles in EC had been the subject of extensive research in the current and past decades. However, androgen receptor (AR) did not get similar research interest until recently.3,6,7
AR is a well-known steroid receptor with a significant influence on male and female hormone-dependent organs. It is expressed in several tissues, including the uterus, where its role is largely unknown.8 Targeting AR has been suggested to be beneficial for specific subgroups of patients receiving breast cancer treatment.9
Our study explores the expression of AR among different types of EC. It aims to determine whether AR expression can be correlated with prognosis and outcome of disease. Thus, it may be used as adjunct hormonal therapy in cases with a high AR expression level.
Methods
We conducted a retrospective study from January 2010 to December 2019. The study was approved by the institutional research ethics review board committee. A search of pathology laboratory database over the last 10 years was performed for a diagnosis of ‘EC’ obtained from diagnostic surgical procedures including dilatation and curettage (D&C); endometrial biopsy, or hysterectomy, etc. Inclusion criteria for the study included the following: primary EC; documented follow-up data in clinic notes and radiology studies of at least six months; full surgical staging at Jordan University Hospital (total abdominal hysterectomy (TAH), bilateral salpingoopherectomy (BSO), pelvic lymph node excision, and debulking); and no use of neoadjuvant or hormonal therapy before primary surgery. Exclusion criteria included metastatic tumors to the uterus, patients who received hormonal or chemotherapy prior to surgery, and those lost to follow-up.
We identified 52 EC. Patient’s medical records for the selected cases were reviewed to retrieve data regarding age at diagnosis, other gynecological diseases, primary management, follow-up period (FUP), and patient outcome, including any recurrences/metastatic disease, additional therapy given, and deaths. Electronic and paper reports were retrieved to collect data about histopathological tumor histotype, tumor grade, FIGO and malignant tumor stage, pelvic lymph node invasion, and ER status.
Microscopic histopathology slides and corresponding paraffin-embedded tissues (including curettages and hysterectomies) were retrieved for review. The cases were sorted (using the staging surgical specimens) according to conventional morphological and immunohistochemical criteria3 into EC-I (40 cases) and EC-II (12 cases). The grade of EEC is determined by the microscopic appearance of the tumor (including architectural pattern, nuclear features, or both).4 Architectural grade is determined by the extent of tumor solid masses as compared with well-defined tumor glands. However, it is important to exclude masses of squamous epithelium in determining the amount of solid growth. The nuclear grade is determined by an increase in nuclear size and shape, chromatin distribution, and size of the nucleoli. A spectrum encompassing oval small nuclei with evenly dispersed chromatin in grade 1 to markedly large and pleomorphic nuclei, with irregular coarse chromatin, and prominent eosinophilic nucleoli in grade 3. Grade 2 nuclei have features intermediate to grades 1 and 3. Increased mitotic activity and abnormal mitotic figures are used to upgrade the individual tumors independently. EC-II, including ESC and ECCC, are regarded as high grade by definition according to conventional histopathological criteria. FIGO stage reflects the extent of disease at the time of diagnosis. Complete surgical staging requires TAH, BSO, and the assessment of the pelvic and para-aortic lymph nodes. Pathologic staging analysis includes evaluating the depth of myometrial invasion, endocervical stromal involvement, extension to adnexae, extrauterine tissues, and pelvic lymph node involvement. FIGO staging encompasses tumors confined to the endometrium stage IA1 to tumors with distant metastasis beyond the pelvis in stage IVB.7
Two pathologists independently verified the histological typing, FIGO grade, and FIGO stage of all cases.
Staining was performed on paraffin-embedded specimens using anti-AR of Novocastra lyophilized mouse monoclonal antibody (Leica Biosystems Newcastle Ltd, UK), using 1:25 dilution. Results were interpreted and scored using an Olympus light microscope. Only nuclear stain was regarded positive. The staining was evaluated using a semi-quantitative system where both stain intensity (SI) and percentage of positive tumor cells (PP) are considered. SI was graded zero (no stain), one (weak), two (moderate), and three (strong). PP was graded from zero, one (< 10%), two (10–50%), three (> 50%), to four (> 75%). AR histologic score (HS) was then calculated as the average product of SI and PP on five random high power fields at 200× magnification. AR HS = 0 was considered negative, and HS 1–12 were considered positive. HS values from 1–5 were also exploited as level of expression (LE) and defined as low LE; and 6–12 as high LE.10 Scoring was done by two pathologists separately blinded to the typing of the original tumor.
Data were analyzed using SPSS (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). A p-value < 0.050 was considered statistically significant. AR, LE, and HS were analyzed against different clinical and histological parameters including age, menopause, tumor type, histotype, grade, ER, atypical complex hyperplasia (ACH), lymph node status, stage, recurrence, death, and outcome. Associations between groups were evaluated using the Pearson’s chi-square test for categorical variables.
Results
The patients’ age ranged from 31–88 years (mean = 60.3±12.61 years). Forty-two (80.8%) patients were menopausal. The diagnostic surgery included endometrial biopsy (EmBx), D&C, TAH with or without BSO, or debulking. The initial diagnosis was made on EmBx and D&C in 34 cases. As mentioned above, all included cases had full surgical diagnostic and therapeutic procedures at our institute. FUP ranged from 6–112 months (mean = 50.9±25.4 months). Secondary or additional non-surgical treatment was received in 27 (51.9%) patients (including chemotherapy alone in 12, radiotherapy alone in 11, and chemotherapy and radiotherapy in four). Twenty-five patients (48.1%) had no additional treatment. Eleven (21.2%) patients had evidence of recurrent disease or metastasis, with secondary FUP ranging from 4–24 months. By the end of FUP, 48 (92.3%) patients were alive and four patients died (7.7%). Forty patients were alive without disease, eight alive with disease, three died because of the disease, and one died of other causes.
Table 1: Clinico-pathological parameters correlated with androgen receptor (AR) expression.
|
Menopause |
|
|
|
0.086 |
|
No |
10 |
6 (60.0) |
4 (40.0) |
|
|
Yes |
42 |
18 (42.9) |
24 (57.1) |
|
|
EC type |
|
|
|
0.005* |
|
I |
40 |
15 (37.5) |
25 (62.5) |
|
|
II |
12 |
9 (75.0) |
3 (25.0) |
|
|
FIGO grade* |
|
|
|
0.035* |
|
1 |
20 |
6 (30.0) |
14 (70.0) |
|
|
2 |
17 |
7 (41.2) |
10 (58.8) |
|
|
3† |
15 |
11 (73.3) |
4 (26.7) |
|
|
FIGO stage |
|
|
|
0.994 |
|
I |
36 |
16 (44.4) |
20 (55.6) |
|
|
II |
5 |
2 (40.0) |
3 (60.0) |
|
|
III |
8 |
4 (50.0) |
4 (50.0) |
|
|
IV |
2 |
1 (50.0) |
1 (50.0) |
|
|
Associated ACH |
|
|
|
0.594 |
|
No |
44 |
21 (47.7) |
23 (52.3) |
|
|
yes |
8 |
3 (37.5) |
5 (62.5) |
|
|
Histotype |
|
|
|
0.044* |
|
Endometrioid |
40 |
15 (37.5) |
25 (62.5) |
|
|
Serous |
9 |
6 (66.7) |
3 (33.3) |
|
|
Clear cell |
3 |
3 (100) |
0 (0.0) |
|
|
Mixed types I and II |
5 |
3 (60.0) |
2 (40.0) |
|
|
Lymph nodes |
|
|
|
0.110 |
|
Positive |
5 |
4 (80.0) |
1 (20.0) |
|
|
Negative |
47 |
20 (42.6) |
27 (57.4) |
|
|
ER by IHC |
|
|
|
0.284 |
|
Negative |
6 |
4 (66.7) |
2 (33.3) |
|
|
Positive |
46 |
20 (43.5) |
26 (56.5) |
|
|
Recurrence/mets |
|
|
|
0.530 |
|
No |
41 |
18 (43.9) |
23 (56.1) |
|
|
Yes |
11 |
6 (54.5) |
5 (45.5) |
|
|
Patient outcome |
|
|
|
0.202 |
|
AWND |
40 |
18 (45.0) |
22 (55.0) |
|
|
AWD |
8 |
3 (37.5) |
5 (62.5) |
|
|
DOD |
3 |
3 (100) |
0 (0.0) |
|
*Grading applies to endometrioid type only, as type II are considered grade three by default.
†Including six cases of type I-EC.
EC: endometrial carcinomas; FIGO: International Federation of Gynecology and Obstetrics; ACH: atypical complex hyperplasia; ER: estrogen receptor; IHC: immunohistochemistry; mets: metastasis; AWND: alive with no disease; AWD: alive with disease; DOD: dead of disease; DOC: dead of other causes.
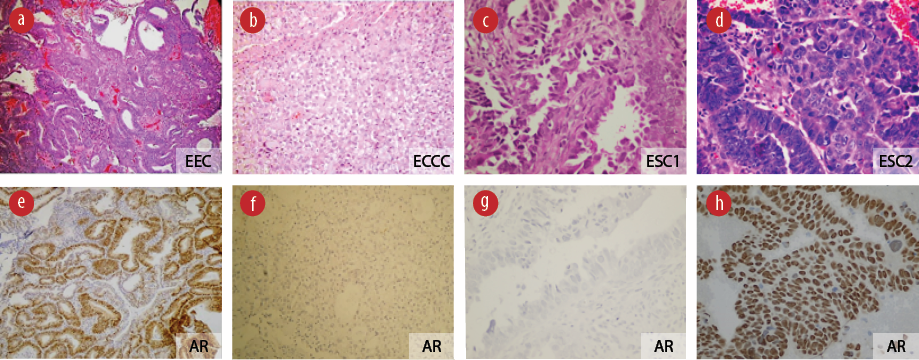
Figure 1: AR expression patterns in different types of endometrial cancer. Upper panel representative cases: (a) EEC: endometrial endometrioid carcinoma; (b) ECCC: endometrial clear cell carcinoma; (c), (d) Endometrial serous carcinoma (ESC) 1 and 2: two different cases of endometrial serous carcinoma (hematoxylin and eosin stain, magnification = 100 ×). (e-h) Lower panel displays corresponding AR expression by immunohistochemical stain, positivity indicated by the brown color (anti-AR, magnification = 100 ×).
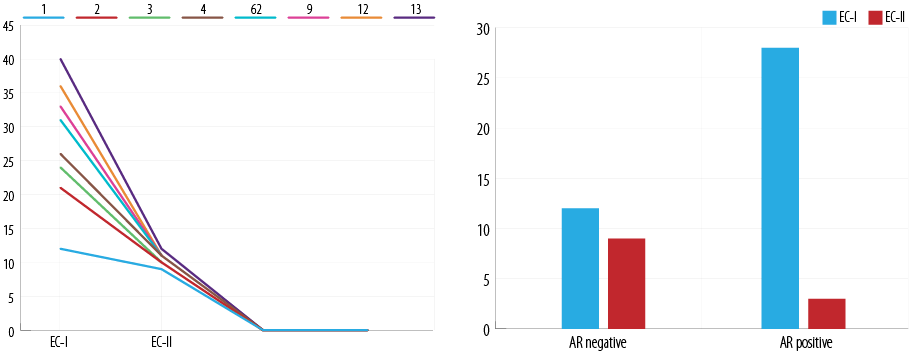
Figure 2: Relationship between androgen receptor (AR) expression and level of expression with endometrial cancer (EC) grade.
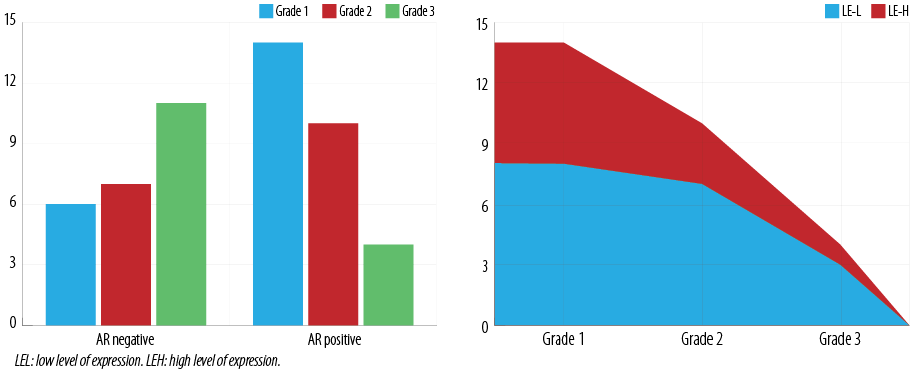
Figure 3: Relationship between androgen receptor (AR) expression and histologic score with the type of endometrial carcinomas (EC).
The histopathological diagnoses of the specimens included 40 EC-I (all were EEC). EC-II consisted of 12 cases (divided into nine ESC and three ECCC). According to tumor grades: 20 (38.5%) were grade 1, 17 (32.7%) were grade 2, and 15 (28.8%) were grade 3. All EC-II were given grade 3 by definition. FIGO staging were: stage I in 36 (69.2%), stage II in five (9.6%), stage III in eight (15.4%), and stage IV in two (3.8%) [Table 1]. Pelvic lymph node involvement was present in five (9.6%) cases.
AR expression by immunohistochemistry was absent in 24 (46.2%) cases and present in 28 (53.8%) cases. Among the AR-positive cases, low expression was seen in 18 (64.3%) cases and 10 (35.7%) cases had high expression [Figure 1].
Out of the entire study sample, 10 patients were non-menopausal, of which four (40.0%) EC had AR positivity. Forty-two patients were menopausal, 24 (57.1%) of which had AR positivity, and 16 (88.9%) revealed low LE. Pearson’s chi-square tests for menopause with AR, low LE, and HS were 0.328, 0.527, and 0.588, respectively.
AR positivity was present in 25 (62.5%) cases of EC-I and three (25.0%) cases of EC-II (p = 0.005) [Figure 2]. The mean AR HS was 3.3±3.9 in EC-I, and 0.3±3.6 in EC-II (Fisher’s exact test = 0.041). Pearson’s chi-square correlations between the tumor type and AR, LE, and HS were performed and the results were 0.005, 0.021, and 0.206, respectively.
Twenty EC were of grade 1 of which 14 (70.0%) were AR positive. Seventeen EC were grade 2, of which 10 (58.8%) were AR positive. Fifteen EC were grade 3, of which four (26.7%) were AR positive [Figure 3]. Pearson’s chi-square correlation coefficient was computed to assess the relationship between the grade of EC and AR, LE, and HS. The results were 0.035, 0.037, and 0.107, respectively. Overall, there was a weak negative correlation between the grade of EC and AR expression.
The histopathological diagnoses of the specimens included 40 EC-I (all were EEC). EC-II consisted of 12 cases (divided into nine ESC and three ECCC). Pearson’s chi-square correlation coefficient was computed to assess the relationship between the histotype of EC and AR, LE, and HS. The results were 0.044, 0.182, and 0.855, respectively.
Pearson’s chi-square correlation coefficient was computed to assess the relationship between the stage of EC and AR, LE, and HS. The results were 0.994, 0.848, and 0.629, respectively.
Five EC cases had positive pelvic lymph nodes, of which only one (20.0%) showed AR positivity, with a low LE. Pearson’s chi-square correlation coefficient was computed to assess the relationship between the pelvic lymph node status and AR, LE, and HS. The results were 0.110, 0.244, and 0.827, respectively.
Of the entire study sample, 11 (21.2%) cases had documented recurrence/metastasis during follow-up. Of those, five (45.4%) cases displayed AR positivity in the primary tumor, and specifically, three had a low LE. Cases without recurrence/metastasis showed AR positivity in the primary tumors in 23 out of 41 (56.1%). Pearson’s chi-square correlation coefficient was computed to assess the relationship between the recurrence of EC and AR, LE, and HS. The results were 0.530, 0.803, and 0.770, respectively.
Four deaths were documented in the study sample. Three (75.0%) deaths were linked to EC complications and progression, while 25.0% were death for unrelated causes. Three deaths were in a patient with EC-I patients and one in an EC-II patient. One patient (100%) had AR-positive EC. Pearson’s chi-square correlation coefficient was computed to assess the relationship between death and AR, LE, and HS. The results were 0.228, 0.421, and 0.582, respectively. As expected, a significant correlation (p = 0.050) is present between advanced tumor stage and death, two cases were stage 3 and one was stage 4.
Eight (15.4%) cases had ACH along with the EC, all of which were EC-I (100%). Of those with ACH, five (62.5%) were AR-positive. Pearson’s chi-square was computed to assess the relationship between the existence of ACH and AR, LE, and HS. The results were 0.396, 0.468, and 0.334, respectively.
Twenty-five patients did not require any additional therapy following the diagnostic surgical procedure. Seventeen (68.0%) had AR positivity in their primary tumors. AR positivity was also detected in six (50%) cases that required chemotherapy, six (54.5%) cases that received radiotherapy, and four (100%) cases that required both chemo and radiotherapy. Pearson’s chi-square correlation coefficient was computed to assess the relationship between the treatment of EC and AR, LE, and HS were 0.279, 0.336, and 0.416, respectively.
Forty-six (88.5%) cases were positive while six (11.5%) were negative for ER expression. Interestingly, of the 46 ER-positive cases, 26 (56.5%) were also AR-positive. Pearson’s chi-square was computed to assess the relationship between ER expression and AR, LE, and HS. The results were 0.397, 0.529, and 0.604, respectively.
Discussion
Sex hormones, mainly estrogens, in gynecological diseases had been extensively explored. For instance, ER positivity in ovarian epithelial tumors was associated with greater disease-free survival. However, there was no significant association between steroid receptor expression and overall survival.11
Conversely, some studies revealed that PR, ER, and AR were detected in the normal ovaries of postmenopausal women in stroma, ovarian surface epithelium, and epithelial inclusion cysts. The expression of PR and AR did not change over time, while the expression of ER decreased following menopause, and it was still detected in patients > 10 years after menopause.11
The role and expression of ER and PR in endometrial cancer have been extensively studied.12 ER and/or PR positivity in primary tumors is associated with well-differentiated lesions and a more favorable prognosis.7 Hormonal therapy targeting both PR and ER is used in the treatment of endometrial cancer. The response rate to such treatments is usually low.7 However, patients expressing hormone receptors are more sensitive to hormonal therapy.13 From a practical point of view, although the available limited clinical trials have shown modest response rates, they have consistently identified a small subset of patients that respond very well to hormonal therapy with few side effects. Recent papers proposed that it is time to perform additional well-designed trials that should include hormonal biomarkers in treating cancers.14
The role of the male-counterpart sex hormones (androgen through its receptor) had been modestly evaluated in females. However, some studies were able to demonstrate a link with tissue expression in normal, benign, and malignant female conditions.15,16 For example, elevated circulating levels of free testosterone correlated with EC risk but not androstenedione or dehydroepiandrosterone sulfate (DHEAS).17 Free testosterone and DHEAS correlate with abdominal fat accumulation, a risk factor for EC, in postmenopausal women.18 Women with polycystic ovary syndrome (PCOS) have a four-fold increased risk of EC (especially EC-I) than non-PCOS women.16 Many patients with symptoms of PCOS have elevated circulating concentrations of androgens that may be associated with the development of hirsutism.16
There have been inadequate studies illuminating the expression of AR in EC. Moreover, the available papers had some contradictory results. Immunostaining for AR in epithelial cells in a small set of grade II EC has been demonstrated.15 One study compared the expression of AR, the more biologically potent form of 5α-reductase types one and two, in 44 cases of EC, and both had shown positivity (88.6 and 80%, respectively).19 In contrast, another study found only 21% positivity among some types of EC.20
From a molecular perspective, a potential role of AR in EC can be proposed through several mechanisms. Androgen-dependent signaling is known to affect the expression of oncogenes, tumor suppressor genes, cell cycle regulators, and metastasis-associated genes that may affect disease progression in many disease conditions, both in males and females.4
In fact, examples from gynecological diseases are emphatically cumulative. For instance, some researchers had found that AR seems not to induce proliferation of endometrium in postmenopausal women and may antagonize the effect of estrogen E2.6 Expression of cyclin D1 as a regulator of cell cycle and proliferation in breast cancer is said to be regulated by AR.21 Liang et al,22 revealed a similar role of cyclin D1 as a prognostic marker in EC. Moreover, activating Kras mutations have been identified in precursor lesions for EC.23 A recent integrated analysis of Kras copy-number alterations and mutations found that increased Kras copy number and mRNA expression, but not Kras mutations, were associated with EC disease progression and poor disease-specific survival. Interestingly, AR signaling was found to decrease Kras protein expression in breast cancer cells.24
In another perspective, androgen can be aromatized to active estrogens. Thus with a greater aromatase enzyme activity, it may act as a prohormone that increases high-risk estrogen exposure, especially in postmenopausal and obese women.8
Furthermore, ‘cross-talk’ between AR and ER signaling due to overlapping binding sites within DNA had been previously reported in breast cancer25 and normal breast tissue.21
As mentioned above, improving our understanding of the role of AR and AR signaling may guide us to an innovative therapeutic approach in EC. Recently, second-generation anti-androgens are developed by computational pharmacophore modeling and virtual screening and have been utilized on different types of cancers, including ovarian cancer.26 There is a growing obligation for well-controlled clinical trials using such regimens in endocrine therapy of EC. In addition, the innovative creation of selective androgen receptor modulators in the management of prostate cancer,27 although not yet proved for gynecological cancer therapy, could be of great potential assistance in the treatment of EC in the future.
Recent studies used tissue microarray to validate their results regarding AR in EC and EC precursor lesions. Expression of AR was observed in 93% of endometrial hyperplasia but only 41% of non-endometrioid tumors.7 The authors of the same study found that AR is more commonly expressed in metastatic lesions compared to ER and PR, and AR status is discordant in primary and metastatic lesions in a large proportion of cases. In another recent study by the same group, AR protein level was significantly associated with survival.28
Our results contradict a newly published paper performed on an endometrial tissue microarray containing 50 EC with a variety of morphologic subtypes as well as 20 benign and nine atypical hyperplastic endometrial tissues, which had shown that high-level expression is seen in half of ESC and carcinosarcoma (also considered EC type II).29
On the other hand, our study revealed similar findings to Mahdi et al,30 who concluded that although not significant, AR expression showed more frequent association with EC-I, early tumor stage (I–II), and low FIGO grade (1–2) EC. Unlike our study, they found that AR expression was significantly correlated with the absence of lymphovascular invasion and decreased LN involvement. Patients with AR expression showed increased disease-free survival and late disease recurrence. In addition, AR expression had a positive, significant correlation with PR and ER expression.30 This study displays a significant correlation between EC-I and immunohistochemical expression of AR. We also found that AR expression was correlated with lower tumor grade and specific histotype. This study, however, failed to detect significant correlations between AR expression parameters and age, menopause, tumor stage, lymph node status, ER expression, ACH, recurrence, outcome, and death.
The strength of the current study arises from the following facts. First, it is the first of its kind in our country and part of the world in that it specifically explored the expression and impact of a hormone receptor that did not get enough literature attention. Second, we performed it at a major tertiary clinical center with well-recognized gynecological, radiological, and histopathological services at the national and international levels. Third, the interfering effect on any hormonal therapy or chemotherapy was excluded by limiting the study criteria to cases without neoadjuvant therapy before diagnostic and therapeutic surgery.
On the other hand, our study also had several limitations, including the small sample size, especially EC type II, and the lack of supportive molecular tests. In addition, it did not include an examination of AR expression in precursor lesions of EC type I and II (endometrial intraepithelial neoplasia or endometrial intraepithelial carcinoma, respectively).
Conclusion
Recent epidemiological, molecular, and therapeutic cell and animal studies suggest a significant role for AR in proliferation, oncogenes expression, cell cycle regulators, tumor suppressor genes, and metastasis-associated genes in many types of cancers.
The impact of AR in EC is not fully understood, as literature about AR action in gynecological tumors is inadequate, unlike those done on female sex hormone receptors. Moreover, some contradiction exists between the results of the limited available papers.
Contemporary efforts, including our study, had demonstrated that AR is expressed in a good proportion of EC, especially type I. It also points out that AR expression may be associated with a lower grade and better differentiated EC.
Future and more comprehensive studies on different populations are required to clarify the literature controversy and explore the potential benefits of AR as a target in hormonal therapy in EC with the appropriate hormonal profile, with the potential benefits of improving patient care.
Disclosure
The authors declared no conflicts of interest. This study was supported by the scientific research deanship at the University of Jordan.
references
- 1. Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the human development index (2008-2030): a population-based study. Lancet Oncol 2012 Aug;13(8):790-801.
- 2. Emons G, Heyl W. Hormonal treatment of endometrial cancer. J Cancer Res Clin Oncol 2000 Nov;126(11):619-623.
- 3. Matias-Guiu X, Catasus L, Bussaglia E, Lagarda H, Garcia A, Pons C, et al. Molecular pathology of endometrial hyperplasia and carcinoma. Hum Pathol 2001 Jun;32(6):569-577.
- 4. Lewin SN. Revised FIGO staging system for endometrial cancer. Clin Obstet Gynecol 2011 Jun;54(2):215-218.
- 5. Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al; Australian National Endometrial Cancer Study Group. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013 Jul;31(20):2607-2618.
- 6. Zang H, Sahlin L, Masironi B, Eriksson E, Lindén Hirschberg A. Effects of testosterone treatment on endometrial proliferation in postmenopausal women. J Clin Endocrinol Metab 2007 Jun;92(6):2169-2175.
- 7. Tangen IL, Werner HM, Berg A, Halle MK, Kusonmano K, Trovik J, et al. Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. Eur J Cancer 2014 Nov;50(17):3003-3010.
- 8. Gibson DA, Simitsidellis I, Collins F, Saunders PT. Evidence of androgen action in endometrial and ovarian cancers. Endocr Relat Cancer 2014 Aug;21(4):T203-T218.
- 9. Fioretti FM, Sita-Lumsden A, Bevan CL, Brooke GN. Revising the role of the androgen receptor in breast cancer. J Mol Endocrinol 2014 Jun;52(3):R257-R265.
- 10. Qiu M, Bao W, Wang J, Yang T, He X, Liao Y, et al. FOXA1 promotes tumor cell proliferation through AR involving the Notch pathway in endometrial cancer. BMC Cancer 2014 Feb;14(1):78.
- 11. Brodowska A, Laszczyńska M, Starczewski A, Brodowski J, Masiuk M, Domagala W. Immunohistochemical analysis of steroid receptors in ovaries of postmenopausal women–effects of aging and hormone status. Histol Histopathol 2010 Aug;25(8):1009-1016.
- 12. Fleming GF, Filiaci VL, Hanjani P, Burke JJ, Davidson SA, Leslie KK, et al. Hormone therapy plus temsirolimus for endometrial carcinoma (Ec): gynecologic oncology group trial #248. J Clin Oncol 2011; 29(15_suppl):5014.
- 13. Wik E, Ræder MB, Krakstad C, Trovik J, Birkeland E, Hoivik EA, et al. Lack of estrogen receptor-α is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clin Cancer Res 2013 Mar;19(5):1094-1105.
- 14. Kokka F, Brockbank E, Oram D, Gallagher C, Bryant A. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database of Systematic Reviews 2010;(12):CD007926.
- 15. Horie K, Takakura K, Imai K, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Hum Reprod 1992 Nov;7(10):1461-1466.
- 16. Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM, Grp AO, et al; Australian Ovarian Cancer Study Group and Australian National Endometrial Cancer Study Group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case-control study. Cancer Causes Control 2010 Dec;21(12):2303-2308.
- 17. Dossus L, Lukanova A, Rinaldi S, Allen N, Cust AE, Becker S, et al. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort–a factor analysis. Am J Epidemiol 2013 Apr;177(8):787-799.
- 18. Cao YK, Zhang SF, Zou SE, Xia X. The relationship between endogenous androgens and body fat distribution in early and late postmenopausal women. PLoS One 2013;8(3):e58448.
- 19. Ito K, Suzuki T, Akahira J, Moriya T, Kaneko C, Utsunomiya H, et al. Expression of androgen receptor and 5alpha-reductases in the human normal endometrium and its disorders. Int J Cancer 2002 Jun;99(5):652-657.
- 20. Sasaki M, Oh BR, Dharia A, Fujimoto S, Dahiya R. Inactivation of the human androgen receptor gene is associated with CpG hypermethylation in uterine endometrial cancer. Mol Carcinog 2000 Oct;29(2):59-66.
- 21. Lanzino M, Sisci D, Morelli C, Garofalo C, Catalano S, Casaburi I, et al. Inhibition of cyclin D1 expression by androgen receptor in breast cancer cells–identification of a novel androgen response element. Nucleic Acids Res 2010 Sep;38(16):5351-5365.
- 22. Liang S, Mu K, Wang Y, Zhou Z, Zhang J, Sheng Y, et al. CyclinD1, a prominent prognostic marker for endometrial diseases. Diagn Pathol 2013 Aug;8(1):138.
- 23. Dobrzycka B, Terlikowski SJ, Mazurek A, Kowalczuk O, Niklińska W, Chyczewski L, et al. Mutations of the KRAS oncogene in endometrial hyperplasia and carcinoma. Folia Histochem Cytobiol 2009;47(1):65-68.
- 24. Lyu S, Yu Q, Ying G, Wang S, Wang Y, Zhang J, et al. Androgen receptor decreases CMYC and KRAS expression by upregulating let-7a expression in ER-, PR-, AR+ breast cancer. Int J Oncol 2014 Jan;44(1):229-237.
- 25. Need EF, Selth LA, Harris TJ, Birrell SN, Tilley WD, Buchanan G. Research resource: interplay between the genomic and transcriptional networks of androgen receptor and estrogen receptor α in luminal breast cancer cells. Mol Endocrinol 2012 Nov;26(11):1941-1952.
- 26. Voet A, Helsen C, Zhang KY, Claessens F. The discovery of novel human androgen receptor antagonist chemotypes using a combined pharmacophore screening procedure. ChemMedChem 2013 Apr;8(4):644-651.
- 27. Gao W, Dalton JT. Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs). Drug Discov Today 2007 Mar;12(5-6):241-248.
- 28. Tangen IL, Onyango TB, Kopperud R, Berg A, Halle MK, Øyan AM, et al. Androgen receptor as potential therapeutic target in metastatic endometrial cancer. Oncotarget 2016 Aug;7(31):49289-49298.
- 29. Zadeh SL, Duska LR, Mills AM. Androgen receptor expression in endometrial carcinoma. Int J Gynecol Pathol 2018 Mar;37(2):167-173.
- 30. Mahdi Z, Abdulfatah E, Pardeshi V, Hassan O, Schultz D, Morris R, et al. The impact of androgen receptor expression on endometrial carcinoma recurrence and survival. Int J Gynecol Pathol 2017 Sep;36(5):405-411.