Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of cancer-related deaths. Common etiologies for HCC include hepatitis B, chronic hepatitis C, cirrhosis of any underlying etiology, and hereditary hemochromatosis. Although HCC is the most common primary hepatic malignancy, the risk of extra-hepatic metastases is relatively low compared to other primary hepatic malignancies. The most common sites of malignant spread by HCC are the lungs, followed by intra-abdominal lymph nodes, bones, and adrenal glands.1,2 Involvement of intra-thoracic lymph nodes is rare. In this report, we present a case of poorly differentiated HCC found to demonstrate metastatic involvement of the prepericardial lymph nodes.
Case report
A 62-year-old female presented to the emergency department (ED) with one week of progressively worsening abdominal pain and distension. Her pain was located in the left upper quadrant, radiated to her back, was 10/10 in severity, and sharp in quality. Her pain was aggravated by lying down or rising from bed and alleviated by staying still. Review of systems was positive for one week of constipation and one episode of non-bloody, non-bilious vomiting three days before presentation, and an episode of hematuria without dysuria on the day of presentation. She presented to her primary care physician four days before arriving at the ED, and was prescribed acetaminophen, ciprofloxacin, and docusate with minimal interval improvement.
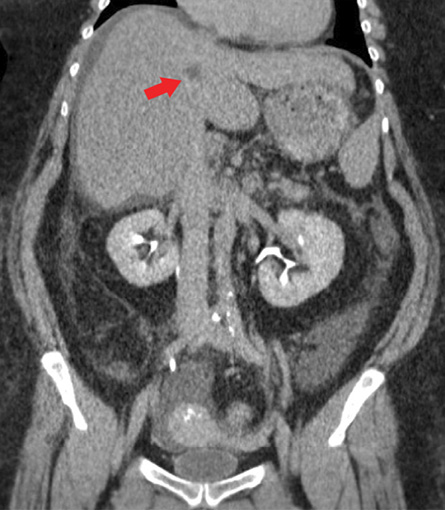
Figure 1: Coronal imaging of a contrast-enhanced abdomen and pelvis CT during the delayed-phase demonstrates subtle nodularity of the liver contour suggestive of cirrhosis. A nonspecific hypodense lesion in the medial portion of the left hepatic lobe, likely in hepatic segment IV, is seen on the delayed-phase that was not conspicuously present on either arterial- or venous-phase (red arrow). Moderate free fluid in the abdomen and pelvis was present and can be seen in the right subphrenic space and Morrison’s pouch in this image.
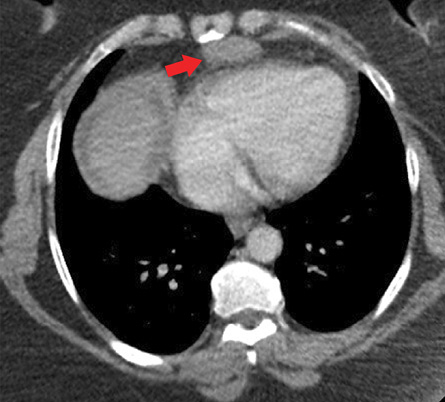
Figure 2: Transverse imaging of a contrast-enhanced CT chest demonstrates a well-marginated homogeneous soft-tissue density mass measuring 3.5 × 1.7 cm in the mediastinal fat anterior to the right ventricle and the region of the prepericardial lymph nodes (red arrow).
Her medical history was significant for chronic hepatitis C diagnosed two years prior, hypothyroidism, chronic obstructive pulmonary disease, hypertension, type II diabetes mellitus, and osteoarthritis of bilateral knees. Of note, she does not have a documented prior history of varices or gastrointestinal bleeding. Past surgical history was significant for a percutaneous liver biopsy performed two years prior, which was reported by the patient to be benign. She had a 30-pack year history of smoking. She denied alcohol or intravenous drug use. The patient reported nonadherence to hepatitis C treatment at the time of presentation.
Physical exam was notable for mild distress, but the patient was alert and oriented and responding appropriately. There was mild scleral and oral mucosal icterus. The abdomen was markedly distended, firm to touch, and dull to percussion in all four quadrants. There were normal bowel sounds on auscultation, and there was no pain on superficial palpation. Significant pain was elicited with deep palpation in the left upper quadrant, but no rebound tenderness or guarding was present. No asterixis was present. There was no appreciable caput medusa, clubbing, palmar erythema, or spider telangiectasias.
Pertinent laboratory tests included an alanine aminotransferase of 483 units/L, aspartate aminotransferase of 139 units/L, CA125 of 233 units/mL, and alpha-fetoprotein of 3673 ng/mL. Carcinoembryonic antigen was within normal limits.
Contrast-enhanced computed tomography (CT) of the abdomen and pelvis during the delayed-phase showed a well-defined 1–2 cm hypodense lesion in hepatic segment IV [Figure 1] that was not conspicuous on either arterial- or venous-phase. Moderate amount of ascites, most notably in the right paracolic gutter, was also present. There was no evidence of gastric, splenic, or esophageal varices. There was no recanalization of the paraumbilical vein, dilatation of the main portal vein, or splenomegaly. Contrast-enhanced CT of the chest showed a well-marginated, homogenous soft-tissue density 3.5 cm substernal mass within the mediastinal fat and in the region of the prepericardial lymph nodes [Figure 2].
Given the concern that the substernal mass was an entity unrelated to the lesion within the liver and rather reflective of a primary mediastinal neoplasm, a cardiothoracic surgeon was consulted, and the resection of substernal mass was ultimately performed. However, the patient, unfortunately, went into fulminant liver failure postoperatively and died due to her complications from liver failure before the final pathology report.
The pathology of the substernal mass revealed metastatic disease by poorly differentiated HCC. The morphologic findings on histopathology combined with the results of immunohistochemistry confirmed the presence of metastatic involvement of a prepericardial lymph node by poorly differentiated HCC [Figure 3]. A Barcelona clinic liver cancer staging was not performed during her hospitalization given the diagnosis of HCC was not yet rendered at the time of her death.
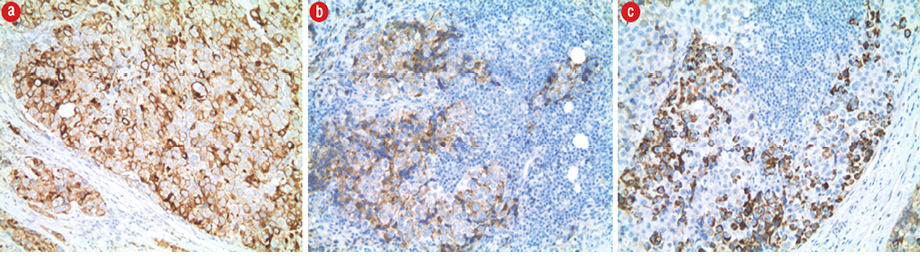
Figure 3: (a) Tumor cells showed strong positivity for low molecular weight cytokeratin CAM 5.2 (CAM 5.2 IHC stain, magnification = 10 ×). (b) Strong positive staining for glypican-3 (glypican-3 IHC stain, magnification = 10 ×). (c) Tumor cells also showed focal positivity for Hepar-1 (Hepar-1 IHC stain, magnification = 10 ×). The tumor cells are negative for vimentin, neuron-specific enolase, chromogranin, CD117, PLAP, HDL, HCG, CD30, desmin, TTF-1, and S100.
Discussion
In developed countries, the most common cause of HCC is cirrhosis, which often occurs in the setting of alcoholic liver disease or hepatitis C. In contrast, in developing countries, the underlying cause is typically chronic hepatitis B and hepatitis C.3 Although HCC is the most common primary hepatic malignancy and frequently associated with intrahepatic spread, the risk of extra-hepatic metastasis is low compared to other primary hepatic malignancies. Specifically, lymph node involvement is relatively uncommon.4 For example, one study reported 28% of autopsy cases with HCC demonstrated lymph node metastasis.5 Another study reported that 12% of metastatic HCC cases demonstrated lymph node involvement, with 4.7% of those cases specifically involving mediastinal lymph nodes.1 In another study, metastatic spread to the mediastinum lymph nodes occurred in 6.5% of cases.2
However, as presented in the current case, solitary involvement of mediastinal lymph nodes in metastatic HCC is extremely rare and reports in the English literature are limited.6,7 The route for intra-thoracic metastasis is thought to be due to lymphatic drainage of the liver through the bilateral triangular ligaments.1 To our knowledge, the first case of mediastinal lymph node metastases by HCC was reported in 1992.8 A limited number of cases of HCC metastasis with specifically substernal lymph node involvement have been reported, some of which occurred in the setting of recurrence,9–12 whereas in other cases, the primary was never found.13,14 Specifically, there was one report of small-sized HCC presenting with large mediastinal metastases.8 While more likely to be related to hematogenous spread, another case reported metastasis to the sternal bone that presented with life-threatening hemorrhage.15
There is equivocal evidence on the optimal approach towards treating HCC with extra-hepatic lymph node metastasis. A multitude of treatment approaches have been previously reported, including surgical resection, video-assisted thoracic surgery (VATS), radiofrequency ablation, percutaneous ethanol injection, and transcatheter arterial chemoembolization (TACE).2,7,16,17 TACE of metastatic mediastinal lymph nodes provided tumor control and increased survival time in one study.17 Chemotherapy is another option with one patient who received 5-fluorouracil/cisplatin and did not demonstrate any detectable metastatic mediastinal lymph nodes following treatment.12 However, the limited number of studies, conflicting findings, and short duration of follow-up of the treatment modalities limit the ability to make substantially meaningful conclusions. Nevertheless, lymph node resection may be a viable option and beneficial in a selected number of cases.5 For example, a report of resection of metastatic mediastinal lymph nodes demonstrated survival of more than 41 months.16 Resection with VATS has been reported to be effective in the treatment of solitary mediastinal lymph node metastasis and cardiophrenic lymph node metastasis following liver resection.4,7 For example, one study reported four months of recurrence-free survival following resection of a solitary mediastinal lymph node metastasis.7
Despite encouraging findings with resection, the presence of lymphatic spread nevertheless portends a poor prognosis.18 As seen in our case, even following resection of a solitary metastatic lymph node, the outcomes in patients with lymphatic spread tend to be relatively poor. In our case, this may be partly attributed to the poor liver function status of the patient at the time of presentation. The prognosis after treatment in patients with lymphatic spread is poorer compared to those with metastases to adrenal glands or the lungs. The cumulative survival rate for extra-hepatic metastasis was reported to be only 21.7% at one year and 7.1% at three years.2 Four variables were identified as significant independent determinants of survival after the initial diagnosis of extra-hepatic metastases: performance status, presence of portal venous invasion, treatment of extra-hepatic metastases, and Child-Pugh grade.2
Conclusion
In summary, we report a case of primary HCC with a solitary mediastinal lymph node metastasis. Given the rare incidence of mediastinal lymph node metastases, the evidence on the most optimal treatment is limited and prognosis is often nevertheless poor.
Disclosure
The authors declared no conflicts of interest.
references
- Katyal S, Oliver JH III, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology 2000 Sep;216(3):698-703.
- Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol 2007 Jan;13(3):414-420.
- Becker AK, Tso DK, Harris AC, Malfair D, Chang SD. Extrahepatic metastases of hepatocellular carcinoma: a spectrum of imaging findings. Can Assoc Radiol J 2014;65(1):60-66.
- 4. Shoji F, Shirabe K, Yano T, Maehara Y. Surgical resection of solitary cardiophrenic lymph node metastasis by video-assisted thoracic surgery after complete resection of hepatocellular carcinoma. Interact Cardiovasc Thorac Surg 2010 Mar;10(3):446-447.
- Hashimoto M, Matsuda M, Watanabe G. Metachronous resection of metastatic lymph nodes in patients with hepatocellular carcinoma. Hepatogastroenterology 2009 May-Jun;56(91-92):788-792.
- Seki S, Kitada T, Sakaguchi H, Nakatani K, Kamino T, Nakamura K, et al. Cardiac tamponade caused by spontaneous rupture of mediastinal lymph node metastasis of hepatocellular carcinoma. J Gastroenterol Hepatol 2001 Jun;16(6):702-704.
- Suzumura K, Hirano T, Kuroda N, Iimuro Y, Okada T, Hashimoto M, et al. Solitary mediastinal metastasis of hepatocellular carcinoma treated by video-assisted thoracic surgery: report of a case. Gen Thorac Cardiovasc Surg 2013 Nov;61(11):651-654.
- Murayama J, Naitoh T, Doi M, Yano H, Ohtsuka M, Yoshizawa Y, et al. [A case of small liver cancer presenting as a huge mediastinal mass]. Nihon Kyobu Shikkan Gakkai Zasshi 1992 Apr;30(4):708-713.
- Nakagawa K, Nakahara K, Ohno K, Matsumura A, Kawashima Y. [Mediastinal dissection of hepatocellular carcinoma with bilateral hilar and mediastinal lymph node metastasis]. Kyobu Geka 1989;42(10):857-860.
- Uchinami H, Abe Y, Kikuchi I, Yoshioka M, Kume M, Sato T, et al. [A case of surgical treatment for solitary lymph node recurrence of hepatocellular carcinoma simultaneously developed in the mediastinum and abdominal cavity]. Nihon Shokakibyo Gakkai Zasshi 2009 Jul;106(7):1049-1055.
- Yamashita R, Takahashi M, Kosugi M, Kobayashi C, Annen Y. [A case of metastatic hepatocellular carcinoma of the superior mediastinum]. Nihon Kyobu Geka Gakkai Zasshi. 1993;41(4):709-713.
- Anami Y, Oguma S, Matsuda Y, Yamaki T, Sazawa Y, Komiya H, et al. [Complete disappearance of metastatic lung tumors and mediastinal lymphnode in a case of hepatocellular carcinoma treated by low-dose 5-fluorouracil/cisplatin therapy]. Gan To Kagaku Ryoho 2005 Nov;32(12):1977-1980.
- Horita K, Okazaki Y, Haraguchi A, Natsuaki M, Itoh T. [A case of solitary sternal metastasis from unknown primary hepatocellular carcinoma]. Nihon Kyobu Geka Gakkai Zasshi. 1996;44(7):959-964.
- Koh PS, Yusof MM, Yoong BK, Rajadurai P. Mediastinal hepatocellular carcinoma with unknown primary: an unusual and rare presentation. J Gastrointest Cancer 2014 Dec;45(Suppl 1):74-76.
- Chen CY, Chau GY, Yen SH, Hsieh YH, Chao Y, Chi KH, et al. Life-threatening haemorrhage from a sternal metastatic hepatocellular carcinoma. J Gastroenterol Hepatol 2000 Jun;15(6):684-687.
- Utsumi M, Matsuda H, Sadamori H, Shinoura S, Umeda Y, Yoshida R, et al. Resection of metachronous lymph node metastases from hepatocellular carcinoma after hepatectomy: report of four cases. Acta Med Okayama 2012;66(2):177-182.
- Chen CC, Yeh HZ, Chang CS, Ko CW, Lien HC, Wu CY, et al. Transarterial embolization of metastatic mediastinal hepatocellular carcinoma. World J Gastroenterol 2013 Jun;19(22):3512-3516.
- Uenishi T, Hirohashi K, Shuto T, Kubo S, Tanaka H, Sakata C, et al. The clinical significance of lymph node metastases in patients undergoing surgery for hepatocellular carcinoma. Surg Today 2000;30(10):892-895.