While pulmonary hypertension (PH) is a well-recognized complication of diffuse parenchymal lung disease (DPLD), the prevalence varies in published studies. Prior reviews suggest that PH is present in 21% of patients with interstitial lung disease (ILD) associated with connective tissue disease,1,2 and the percentage may be even higher with advanced, end-stage idiopathic pulmonary fibrosis (IPF).3 The risk of developing PH is much higher in combined pulmonary fibrosis and emphysema syndrome compared to IPF alone, with estimates approaching 50–90%.4,5 PH is also commonly observed in patients with various forms of respiratory disorders with a mixed restrictive and obstructive pattern who experience chronic hypoxemia.6 Furthermore, PH associated with chronic lung disease is a significant predictor of increased mortality.7 The common occurrence and prognostic significance emphasize the importance of simple measures to detect PH in DPLD patients.
Both DPLD and PH share common respiratory symptomatology; therefore, the diagnosis of PH may be missed in DPLD patients until signs of right heart failure develop.3 Right heart catheterization (RHC) is the diagnostic gold standard for PH, but it is invasive and routine use to screen all DPLD patients is not practical. An echocardiogram (ECHO) is the standard screening test,8 but would add a significant cost of care to perform on every DPLD patient. A more readily available, lower cost, and noninvasive means of screening is preferable.9 Identification of reliable indicators of PH in tests that are routinely performed in DPLD could provide a basis for clinicians to identify appropriate patients for further testing with ECHO or RHC as recommended by the Fifth World Health Symposium on Pulmonary Hypertension.10
Past work has demonstrated that IPF patients with PH had a significantly lower 6-minute walk distance (6MWD).11,12 One study with 199 patients with mixed types of ILD found that patients with a 6MWD below 345 m had a higher chance of having PH after correcting for lung function parameters and IPF (OR 4.9, range 1.68–14.3, p = 0.004).12 The reduced walk distance was presumably due to the PH and associated right ventricular (RV) dysfunction superimposed on the respiratory limitation imposed by ILD alone.
The purpose of the study was to explore the influence of a 6-minute walk test (6MWT) as a simple, non-invasive tool to detect RV dysfunction in patients with DPLD. If the 6MWT was found to be reliable, the results could be used to identify patients in whom ECHO would be appropriate to screen for PH.
Methods
The study was approved by the Mayo Clinic Institutional Review Board (IRB). We retrospectively reviewed the medical charts of patients who were evaluated in the PH Center at the Mayo Clinic in Jacksonville, Florida, from January 1999 to December 2014. Only those with a World Health Organization (WHO) diagnostic group 3 (PH due to lung disease) were included.13 The analysis was limited to the patients with DPLD. Demographics, functional class, clinical history of ILD, chest computed tomography, pulmonary function test (PFT), ECHO results including RV size, function, RV systolic pressure (RVSP), mean pulmonary arterial pressure (MPAP), tricuspid annular plane systolic excursion (TAPSE), and RV index of myocardial performance (RIMP) were examined.
RV systolic dysfunction was defined as one of the following14:
- Qualitative visual assessment showed dysfunction.
- Quantitative measured TAPSE less than 16 mm.
- Quantitative measured RIMP more than 0.40 using the pulse Doppler method.
The 6MWT was performed according to the American Thoracic Society guidelines.15 Pulse oximetry during rest and exertion, oxygen supplementation, total distance walked or 6MWD, and the difference between peak heart rate and heart rate one-minute post-recovery was collected.
A waiver of consent for a minimal risk study was requested and approved by the IRB. The data was collected from the medical charts without communicating to or contacting the patients.
Descriptive statistics were used to analyze the patient characteristics. Normally distributed continuous data were described as mean and standard deviation and tested with the Student’s t-test. The Mann-Whitney U-test was used if the data was not normally distributed. The categorical variables are reported as a percentage of total subjects and compared to the chi-square test. Correlation analysis was used to identify the relationship between 6MWD and ECHO-derived parameters (TAPSE, RIMP, and PFT) results. A p-value < 0.050 was considered statistically significant. The ability of 6MWD to predict RV dysfunction was assessed by logistic regression analysis. Odds ratios (OR) with 95% confidence interval (CI) were used. The data was analyzed using SPSS Statistics (SPSS Statistics, Chicago, US) version 16.
Results
There were 201 patients with WHO group 3 PH. Only those with PH due to DPLD were included. The diagnosis was based on typical imaging and supporting investigations or tissue pathology. Most of the patients were diagnosed with IPF. The second most common diagnosis was combined pulmonary fibrosis and emphysema syndrome. Seventeen patients (35%) had histopathological data available (14 explants, 3 surgical lung biopsies). The characteristics of the patients, histopathological findings, and multidisciplinary diagnoses are listed in Table 1 and 2. We excluded the patients without both available 6MWT and ECHO data, leaving 48 patients in the study.
Table 1: Baseline demographics of all patients (n = 48).
|
Gender (female) |
20 (41.7) |
|
Age, years, mean ± SD, (range) |
65.0±13.9 (19–82) |
|
WHO functional class |
|
|
2 |
5 (10.4) |
|
3 |
33 (68.8) |
|
4 |
10 (20.8) |
|
Pulmonary function test,
mean ± SD, (range) |
|
|
FEV1, L |
1.75± 0.6 (0.3–3.4) |
|
FEV1, % |
62±21 (17–109) |
|
FVC, L |
2.28±0.9 (0.6–4.7) |
|
FVC, % |
63±23 (25–117) |
|
TLC, % |
67±20 (30–116) |
|
DLCO, L |
7.4±2.9 (3.9–15) |
|
DLCO, % |
31.2±12.0 (11–64) |
|
Chest CT pattern (n = 47) |
|
|
Emphysema |
17 (36.2) |
|
Ground glass opacity |
8 (17.0) |
|
Mosaic attenuation |
4 (8.5) |
|
Pleural plaque |
1 (2.1) |
|
Reticular opacities |
20 (42.6) |
|
Honeycombing |
26 (55.3) |
DLCO: diffusing capacity of the lungs for carbon monoxide; FEV1: forced expiratory volume; FVC: forced vital capacity; ILD: interstitial lung disease; TLC: total lung capacity; WHO: World Health Organization.
Thirty-eight patients (79.2%) had preserved FEV1/FVC ratio with mean FEV1 of 63±20% with mild restriction (total lung capacity (TLC) 63±17%). Ten patients (20.8%) had moderate obstruction with FEV1/FVC ratio less than 70% and a mean FEV1 of 58±27% predicted. Of those with obstruction, the mean TLC was 83±25%. The average predicted diffusing capacity of the lungs for carbon monoxide (DLCO) adjusted for hemoglobin was severely reduced at 31±12% (n = 40/48). Most of the patients were hypoxemic with the mean partial pressure of oxygen (PaO2) of 58±14 (range 32–82, n = 41) on room air. The mean oxygen saturation at rest and after exercise was 87±9% (range 61–99) and 84±5% (range 77–98), respectively. The average 6MWD was severely reduced at 281±102 m. Oxygen supplementation was needed in 41 patients during the walks. Patients’ oxygen requirement was 2–25 L/min (9.2±6.4 L). The oxygen delivery device was changed from a nasal cannula to the different types of masks if they required the higher flow of oxygen, usually 8 L/min or above.
All patients had an ECHO: 25 patients had RV enlargement, and 25 patients had RV dysfunction. RV function was quantitatively assessed in 11 patients, and only three had evidence of RV dysfunction. The average TAPSE was 18.9±7.1 mm (n = 11/48). Only three patients had RIMP documented (0.3±0.2).
Patients with RV dysfunction had significantly greater RVSP and MPAP from ECHO, and MPAP from RHC than those with normal RV function [Table 3].
Table 2: Diagnoses obtained after multidisciplinary review.
|
Idiopathic pulmonary fibrosis |
19 |
UIP (6 explants, 3 surgical biopsies) |
|
Combined pulmonary fibrosis and emphysema |
11 |
UIP/emphysema (2 explants)
UIP/fibrotic NSIP/emphysema (1 explant) |
|
Connective tissue disease-associated ILD |
5 |
NSIP (1 explant) |
|
Chronic hypersensitivity pneumonitis |
3 |
Non-necrotizing granulomatous inflammation with UIP
(2 explants) |
|
Sarcoidosis |
2 |
Non-necrotizing granulomatous inflammation by transbronchial fine needle mediastinal lymph node aspiration |
|
Asbestos, pulmonary fibrosis |
1 |
No surgical biopsy |
|
Radiation related pulmonary fibrosis |
1 |
No surgical biopsy |
|
Surfactant protein C mutation associated ILD |
1 |
NSIP (1 explant) |
|
Unclear etiology |
|
|
|
Possible idiopathic pulmonary fibrosis |
4 |
No surgical biopsy |
ILD: interstitial lung disease; NSIP: nonspecific interstitial pneumonia; UIP: usual interstitial pneumonia.
Table 3: Demographic variables of patients with and without right ventricular dysfunction.
|
Gender, female, n (%) |
9 (36.0) |
11 (47.8) |
0.559 |
|
Age, years |
65±15 |
64±13 |
0.438 |
|
Pulmonary function test |
|
|
|
|
FEV1, % |
64±23 |
59±19 |
0.409 |
|
FVC, % |
65±26 |
61±19 |
0.672 |
|
TLC, % |
70±22 |
64±18 |
0.374 |
|
DLCO, % |
29±12 |
34±11 |
0.040 |
|
FVC/DLCO ratio |
2.6±1 |
2.2±1 |
0.150 |
|
Oxygen saturation |
|
|
|
|
at rest |
86±7.7 |
87±10.9 |
0.522 |
|
with exertion |
84±4.0 |
84±5.4 |
0.478 |
|
6MWT distance, meters |
258±108 |
301±88 |
0.176 |
|
Heart rate at 1-minute recovery, bpm |
24±13 |
24±12 |
0.814 |
|
MPAP from right heart catheterization (n=38), mmHg |
41±9 |
34±8 |
0.016 |
|
RVSP from echocardiogram, mmHg |
68±24 |
52±13 |
0.015 |
DLCO: diffusing capacity of the lungs for carbon monoxide; FEV1: forced expiratory volume; FVC: forced vital capacity; MPAP: mean pulmonary arterial pressure; RSVP: right ventricular systolic pressure; SD: standard deviation; TLC: total lung capacity; 6MWT: six-minute walk test.
Logistic regression revealed no impact of 6MWD on RV dysfunction (OR 0.995; 95% CI 0.980–1.001, p = 0.138). Spearman’s rank correlation showed a significant negative correlation between 6MWD and MPAP from RHC (r = -0.41, p = 0.010), 6MWD and RVSP (r = -0.51, p < 0.001), and 6MWD and MPAP obtained with an ECHO (r = -0.46, p =0.013) [Figure 1]. An assessment of the relationship between 6MWD and PH severity by RHC showed MPAP differed statistically between all groups (p = 0.010). Based on MPAP, the severity of PH is categorized as mild (MPAP = 25–35 mmHg), moderate (35–45 mmHg) and severe (over 45 mmHg).16 The mean 6MWD in patients with severe PH (191±97 m, n = 9) was lower than those with mild (300±99 m, n = 19) and moderate PH (309±64 m, n = 10).
Thirty-two patients (67%) performed the 6MWT and ECHO on the same day whereas nine (19%) had both tests within one week. Five patients (10%) had ECHO performed more than two months apart from the 6MWT. We also found no significant correlation between 6MWD and percent predicted FEV1 (r = 0.03, p = 0.851), FVC (r = 0.02, p = 0.880), TLC (r = 0.09, p = 0.522), DLCO (r = -0.06, p = 0.719), or FVC/DLCO ratio (r = -0.03, p = 0.860).
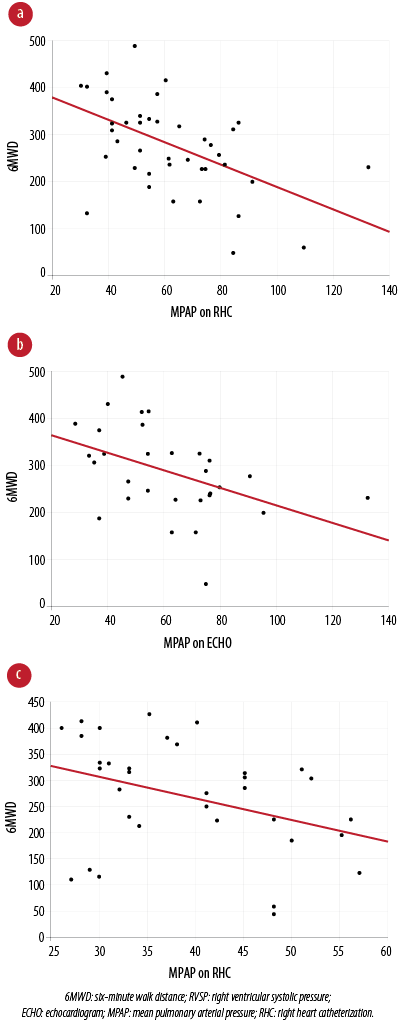
Figure 1: Correlations between 6MWD and the pulmonary hemodynamics. (a) Moderate negative correlation between 6MWD and RVSP on echocardiogram (r = -0.51, p < 0.001). (b) Moderate negative correlation between 6MWD and MPAP on echocardiogram (r = -0.46, p = 0.013). (c) Moderate negative correlation between 6MWD and MPAP on right heart catheterization (r = -0.41, p = 0.010).
Discussion
In contrast to our hypothesis, 6MWD was not useful in this cohort to predict RV dysfunction. The negative result was particularly surprising in light of increased prevalence of RV dysfunction in the study population. Fifty-two percent of patients with DPLD and PH had evidence of RV dysfunction. Furthermore, the patients with RV dysfunction had a higher MPAP from RHC and right heart pressure from ECHO. The patients who had RV dysfunction also had lower percentage predicted DLCO irrespective of the clinical characteristics (including demographics, WHO functional class, and the severity of DPLD based on lung function, i.e., FEV1, FVC, and TLC as percent predicted).
The studies evaluating the RV performance in patients with ILD with and without PH are limited. One study reported the prevalence of RV dysfunction at 66% in patients with severe ILD who were evaluated for lung transplantation.17 In contrast to our study, the severity of the disease correlated to RV systolic dysfunction based on correlations between TAPSE and percent predicted FVC (r = 0.53, p = 0.001), as well as TAPSE and 6MWD (r = 0.50, p = 0.021).17
Pitsiou and colleagues18 reported a worse outcome in clinically stable and ambulatory patients with IPF exhibiting mild to moderate PH with RV systolic dysfunction. RV impairment was defined by a reduction in the ratio of early transtricuspid filling velocity to early diastolic tricuspid annulus velocity (E/Em) below 4.7 (log-rank statistic 5.81, p = 0.016).18
Altered pulmonary hemodynamics had an impact on 6MWD evidenced by the significant negative correlations between 6MWD and right heart pressure. Similar to previous studies, patients with PH had a reduced 6MWD compared to patients without PH.2,11,19 PH had a negative impact on exercise capacity in IPF patients measured by cardiopulmonary exercise parameters, which might not be adequately assessed by resting PFT.20
Nonetheless, a review of previously published work reveals inconsistent reliability. For example, Modrykamien et al21 reported that 6MWD, ECHO, and pulse oximetry performed poorly in detecting PH in IPF patients and suggested RHC for evaluation. Studies evaluating the measurement properties of 6MWD in patients with ILD have been limited by small sample size, including our study, presumably contributing to the inconsistent results.22,23
Interestingly, in patients affected by both ILD and PH, irrespective of the severity of lung disease, 6MWD was similar [Table 2]. None of the PFT parameters (except percent predicted DLCO) differed between the two groups. The small cohort may have been insufficient to reflect the difference in exercise intolerance. Prior studies of 6MWD and the severity of chronic lung disease based on PFT assessment have varied results.11,12,17,20–23
Regardless, the 6MWT remains a practical and reliable measure of exercise tolerance that is widely used to assess the functional status of patients with a variety of cardiac and pulmonary diseases.15,23 Additionally, 6MWD has an advantage as a prognostic value in patients with DPLD. A baseline 6MWD < 250 m and a 24-week decline in 6MWD > 50 m were strong independent predictors of mortality in 748 patients with IPF after controlling for age, respiratory hospitalization, FVC percent predicted, and 24-week change in FVC.24 Lederer et al19 retrospectively reviewed 454 patients with IPF listed for lung transplantation and reported that a lower 6MWD predicted increased mortality and PH when using a threshold of 207 meters. Furthermore, Lama and colleagues25 reported desaturation during 6MWT predicted worse survival in 123 patients with idiopathic interstitial pneumonia (OR 4.49; 95% CI 1.58–12.64, p = 0.005).
Despite recent advances in pharmacological therapy for pulmonary arterial hypertension, the role of specific agents to treat PH in ILD patients is not yet established. Nonetheless, early PH identification in DPLD may permit more aggressive management of the underlying lung disease and identify patients needing lung transplant evaluation. Pulmonary rehabilitation in this particular group may also have a positive impact on functional status and quality of life.26
We acknowledged several limitations to this study. First, our study was limited by the retrospective design and limited sample size. The RV performance was mostly evaluated by qualitative rather than quantitative measures, which may be more accurate. Multiple factors including performing the 6MWT and ECHO on a different day and oxygen supplement during the walk15 could have an impact on the clinical status and 6MWD. Lastly, several patients with DPLD were excluded due to unavailable or incomplete data.
Conclusion
Using a single-center cohort of patients with DPLD with PH, we found that 6MWD was not useful to predict RV dysfunction but did negatively correlate with right heart pressure. In contrast, a severely reduced 6MWD was related to PH defined by both ECHO and RHC; therefore, 6MWT may be used to justify an ECHO to identify patients with poor functional status likely a worse prognosis. Ultimately, a larger prospective study will be required to determine more definitive relationships.
Disclosure
The authors declared no conflicts of interest. No funding was received for this work.
references
- Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J 2007 Oct;30(4):715-721.
- 2. Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006 Mar;129(3):746-752.
- 3. Behr J, Ryu JH. Pulmonary hypertension in interstitial lung disease. Eur Respir J 2008 Jun;31(6):1357-1367.
- 4. Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, et al. Groupe d’Etude et de Recherche sur les Maladies Orphelines Pulmonaires (GERM O P). Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J 2005 Oct;26(4):586-593.
- 5. Mejía M, Carrillo G, Rojas-Serrano J, Estrada A, Suárez T, Alonso D, et al. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest 2009 Jul;136(1):10-15.
- 6. Poor HD, Girgis R, Studer SM. World health organization group iii pulmonary hypertension. Prog Cardiovasc Dis 2012 Sep-Oct;55(2):119-127.
- 7. King TE Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001 Oct;164(7):1171-1181.
- 8. Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013 Dec;62(25)(Suppl):D42-D50.
- 9. Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011 Feb;183(4):431-440.
- 10. Seeger W, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013 Dec;62(25)(Suppl):D109-D116.
- 11. Minai OA, Santacruz JF, Alster JM, Budev MM, McCarthy K. Impact of pulmonary hemodynamics on 6-min walk test in idiopathic pulmonary fibrosis. Respir Med 2012 Nov;106(11):1613-1621.
- 12. Andersen CU, Mellemkjær S, Hilberg O, Nielsen-Kudsk JE, Simonsen U, Bendstrup E. Pulmonary hypertension in interstitial lung disease: prevalence, prognosis and 6 min walk test. Respir Med 2012 Jun;106(6):875-882.
- 13. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013 Dec;62(25)(Suppl):D34-D41.
- 14. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the american society of echocardiography endorsed by the european association of echocardiography, a registered branch of the european society of cardiology, and the canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23:685-713; quiz 786-688.
- 15. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002 Jul;166(1):111-117.
- 16. Chemla D, Castelain V, Hervé P, Lecarpentier Y, Brimioulle S. Haemodynamic evaluation of pulmonary hypertension. Eur Respir J 2002 Nov;20(5):1314-1331.
- 17. Nowak J, Jastrzebski D, Streb W, Rozentryt P, Wojarski J, Greif M, et al. Right ventricular function in patients with severe interstitial lung disease: a Tissue Doppler imaging study. J Physiol Pharmacol 2008 Dec;59(Suppl 6):531-538.
- 18. Pitsiou G, Papadopoulos CE, Karvounis HI, Karamitsos TD, Giannakoulas G, Efthimiadis G, et al. Utility of tissue Doppler imaging in predicting outcome in patients with idiopathic pulmonary fibrosis. Hellenic J Cardiol 2007 May-Jun;48(3):143-151.
- 19. Lederer DJ, Arcasoy SM, Wilt JS, D’Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006 Sep;174(6):659-664.
- 20. Boutou AK, Pitsiou GG, Trigonis I, Papakosta D, Kontou PK, Chavouzis N, et al. Exercise capacity in idiopathic pulmonary fibrosis: the effect of pulmonary hypertension. Respirology 2011 Apr;16(3):451-458.
- 21. Modrykamien AM, Gudavalli R, McCarthy K, Parambil J. Echocardiography, 6-minute walk distance, and distance-saturation product as predictors of pulmonary arterial hypertension in idiopathic pulmonary fibrosis. Respir Care 2010 May;55(5):584-588.
- 22. Chetta A, Aiello M, Foresi A, Marangio E, D’Ippolito R, Castagnaro A, et al. Relationship between outcome measures of six-minute walk test and baseline lung function in patients with interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis 2001 Jun;18(2):170-175.
- 23. du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med 2011 May;183(9):1231-1237.
- 24. du Bois RM, Albera C, Bradford WZ, Costabel U, Leff JA, Noble PW, et al. 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J 2014 May;43(5):1421-1429.
- 25. Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003 Nov;168(9):1084-1090.
- 26. Huppmann P, Sczepanski B, Boensch M, Winterkamp S, Schönheit-Kenn U, Neurohr C, et al. Effects of inpatient pulmonary rehabilitation in patients with interstitial lung disease. Eur Respir J 2013 Aug;42(2):444-453.