Obstructive Sleep Apnea (OSA) is a sleep disorder characterized by repetitive interruptions of breathing during sleep.1,2 OSA with daytime sleepiness is estimated to affect about 3–7% of adult men and 2–5% of adult women, with higher prevalence among groups such as the elderly and those with high body mass index (BMI).2 OSA results from upper airway collapse and is often associated with hypoxia, fragmented sleep, and daytime somnolence.3 Chronic OSA can lead to substantial systemic complications, ranging from cardiovascular and metabolic diseases to neurocognitive impairments.4,5
Secondary polycythemia is a less recognized but clinically significant sequela of OSA.6 This condition, characterized by an abnormal increase in red blood cell (RBC) mass, often results from chronic hypoxemia—a central feature of OSA. During apneic episodes, intermittent oxygen desaturation triggers a compensatory increase in erythropoietin production, which in turn stimulates erythropoiesis.7 While this adaptive response is aimed at improving oxygen delivery, the resultant polycythemia can increase blood viscosity, increasing the risk of thrombotic complications.8
Red cell distribution width, a measure of variability in the size of RBCs, is often elevated in OSA, potentially reflecting the body’s response to chronic intermittent hypoxia.9 Red cell distribution is a helpful parameter in differentiating between secondary and primary erythrocytosis.10
Despite the clear physiological link, the relationship between OSA and secondary polycythemia remains underexplored. Understanding the prevalence, pathophysiology, and clinical implications of this association is essential for early diagnosis and tailored management. Moreover, effective treatment of OSA, particularly with continuous positive airway pressure (CPAP), may have the potential to mitigate polycythemia.
This study aims to delve into the interplay between OSA and secondary polycythemia by assessing the prevalence and severity of OSA in patients with secondary polycythemia. Our findings emphasize the need for comprehensive diagnostic and treatment strategies to prevent downstream complications such as thrombosis.
Methods
A hospital-based retrospective cross-sectional study was conducted among patients attending a hematology clinic in Sultan Qaboos University Hospital, a tertiary teaching hospital in Muscat. The study included all patients who attended the hematology clinic with polycythemia and underwent sleep studies between 2013 and 2023. The study was approved by the ethical committee at the College of Medicine and Health Sciences, Sultan Qaboos University (MREC#3075).
The inclusion criteria were as follows: adult patients (≥ 18 years) diagnosed with secondary polycythemia (elevated RBC count and hemoglobin/hematocrit (Hct) levels) who underwent a sleep study as part of investigations into an underlying condition, such as bone marrow abnormality.11 The exclusion criteria comprised: primary polycythemia,12 smoking, chronic obstructive pulmonary diseases, renal failure, blood cancer, and patients living at high altitudes.
Patient data was collected using the hospital’s TrackCare system. The retrieved demographic data included sex, age, BMI, and blood pressure. Hematological parameters were determined from complete blood count, hemoglobin, hematocrit, mean cell volume, and mean cell hemoglobin.
We also evaluated sleep study data, including the apnea-hypopnea index, baseline oxygen saturation below 90%, desaturation index (DI), and the patient’s subjective report of daytime sleepiness evaluated using the Epworth Sleepiness Scale.
The data was analyzed using the IBM SPSS Statistics (IBM Corp. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp; 2020). Continuous variables were presented as means ± SD and categorical variables as frequencies and percentages. The Chi-square test was used to examine the significance of associations.
Results
After screening 524 patients with polycythemia, 44 patients with secondary polycythemia who had undergone a sleep study were identified and selected for the study. Figure 1 shows the selection process. The patients were overwhelmingly male (42; 95.5%), with only two women. The mean age of the cohort was 40.7 ± 10.6 years, with a mean BMI of 31.7 ± 6.3 kg/m2 [Table 1]. All 44 patients met the diagnostic criteria for secondary polycythemia, and had a mean hemoglobin of 16.5 ± 1.3 g/dL and a mean Hct of 0.5% [Table 1].
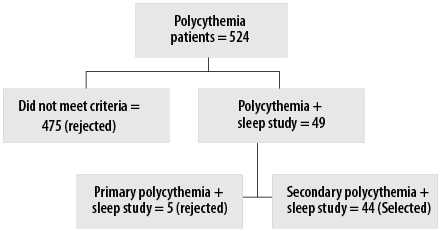 Figure 1: Flow chart of the selection process for secondary polycythemia patients who underwent sleep study.
Figure 1: Flow chart of the selection process for secondary polycythemia patients who underwent sleep study.
Table 1: Demographic and hematological characteristics of patients with secondary polycythemia (N = 44).
|
Age, years
|
40.7 ± 10.6
|
|
Body mass index, kg/m2
|
31.7 ± 6.3
|
|
Systolic blood pressure, mmHg
|
137.0 ± 16.0
|
|
Diastolic blood pressure, mmHg
|
84.0 ± 11.0
|
|
Red blood cells, ×106 /µL
|
6.3 ± 0.9
|
|
Hemoglobin, g/dL
|
16.5 ± 1.3
|
|
Hematocrit, %
|
0.5 ± 0.04
|
|
Mean cell volume, fL
|
80.0 ± 7.8
|
|
Mean cell hemoglobin, pg
|
27.7 ± 9.3
|
|
White blood cells, ×103 /µL
|
6.7 ± 1.9
|
Most participants (52.3%) had severe OSA (mean apnea-hypopnea index: 33.3 ± 23.2), followed by moderate (18.2%) and mild (29.6%) OSA [Figure 2]. Patients with an oxygen saturation index below 90% had a mean OSA of 47.7 ± 24.5 (p = 0.036) [Table 2]. Kurskal-Wallis test revealed that higher Hct levels were significantly associated with severe OSA (p = 0.036) [Figure 3]. Nevertheless, there was no statistically significant association between the severity of OSA and other hematological parameters.
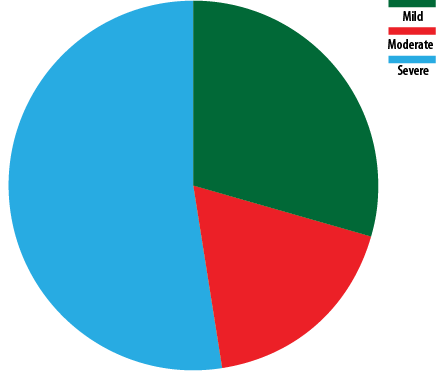 Figure 2: Distribution of severity of obstructive sleep apnea among patients with secondary polycythemia (N = 44).
Figure 2: Distribution of severity of obstructive sleep apnea among patients with secondary polycythemia (N = 44).
Table 2: Characteristics of OSA in patients with secondary polycythemia (N = 44).
|
Epworth sleepiness scale score
|
11 ± 5
|
|
Apnea-hypopnea index, events/hour
|
33.3 ± 23.2
|
|
Baseline SpO2, %
|
94.3 ± 3.4
|
|
Minimum SpO2, %
|
81.1 ± 10.1
|
|
Time with SpO₂ below 90%,
% of total sleep time
|
47.7± 24.5
|
OSA: obstructive sleep apnea; SpO2: peripheral oxygen saturation.
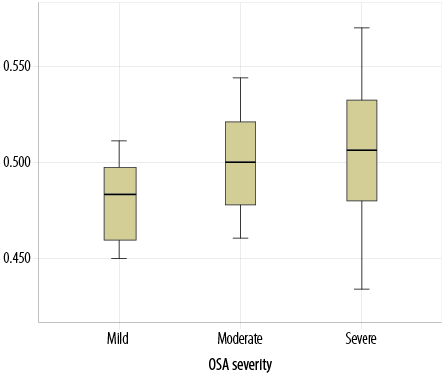 ANOVA: Analysis of Variance; AHI: Apnea-hypopnea index.
ANOVA: Analysis of Variance; AHI: Apnea-hypopnea index.
Figure 3: Analysis of Variance comparison of hematocrit levels (g/dL) across three categories of obstructive sleep apnea (OSA). Hematocrit levels are seen significantly higher in patients with severe OSA (Apnea-hypopnea index (AHI > 30) compared to those with mild OSA (AHI = 5–15) and moderate OSA (AHI = 15–30); p = 0.036.
Table 3 presents the analysis of variance assessing the association between Hct levels and the severity of OSA.
Table 3: ANOVA of the relationship between hematocrit level and severity of OSA in patients with secondary polycythemia (n = 39).
|
Mild
|
12
|
0.48 ± 0.02 (0.45–0.51)
|
0.006
|
0.47–0.49
|
0.036
|
|
Moderate
|
7
|
0.50 ± 0.03 (0.46–0.54)
|
0.010
|
0.47–0.53
|
|
|
Severe
|
20
|
0.51 ± 0.04 (0.43–0.57)
|
0.008
|
0.49–0.53
|
|
ANOVA: analysis of variance; OSA: obstructive sleep apnea.
Discussion
This study assessed the prevalence and severity of OSA among patients with secondary polycythemia. The majority of patients in the study (70.5%) had moderate to severe OSA, which was accompanied by elevated Hct levels. Our results suggest that greater severity of sleep-disordered breathing may have contributed to increased Hct levels, which may have triggered erythrocytosis. This points to a potential correlation between the severity of sleep-disordered breathing and the degree of erythrocytosis. Previous research shows that characteristic intermittent hypoxia associated with OSA triggers erythropoiesis through increased erythropoietin production, leading to increased RCB mass and Hct level.7,8,13 Our results support the existing recommendation that patients presenting with unexplained secondary polycythemia should be screened for OSA.
Several studies have indicated a relationship between OSA severity and Hct levels via increased erythropoietin production.13,14 Our results also emphasize the diagnostic value of sleep study in patients with secondary polycythemia, particularly those with risk factors such as obesity, male sex,15 and a history of excessive daytime sleepiness.
Additionally, we observed a trend in this cohort towards higher BMI and greater neck circumference, both known risk factors for OSA.16 This indicates the multifactorial nature of polycythemia in these patients, where obesity-related hypoventilation may have further exacerbated erythrocytosis.17 The benefit of CPAP therapy in reversing polycythemia has been well-documented, and our findings further emphasize the need for early diagnosis and treatment of OSA in such cases.
Despite the strengths of our study, including a well-defined patient cohort and objective sleep study assessments, several limitations must be acknowledged. First, this was a single-center study with a relatively small sample size, limiting generalizability. Second, the cross-sectional design precluded assessment of causality over the long term. The retrospective nature of data also had potential limitations. Future research should aim to explore the long-term impact of CPAP therapy on Hct levels in secondary polycythemia patients with moderate and severe OSA, as well as the potential genetic predispositions to OSA-related erythrocytosis.
Conclusion
Our study highlights the prevalence of OSA among secondary polycythemia patients diagnosed at a single center in Oman, emphasizing the importance of routine OSA screening in patients presenting with unexplained erythrocytosis. Early identification and treatment of OSA in this population may not only improve their sleep-related quality of life but also reduce hematologic consequences, including the risk of thromboembolic complications associated with chronic hypoxia. It is possible that OSA may contribute to secondary polycythemia in some patients, which warrants further investigation.
Disclosure
The authors declare no conflict of interest. No funding was received for this study.
Acknowledgments
The authors would like to acknowledge the support from the sleep laboratory team at Sultan Qaboos University Hospital.
references
- 1. Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 2001 Mar;163(3 Pt 1):685-689.
- 2. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008 Feb 15;5(2):136-143.
- 3. Al Lawati R, Al Abri MA, Kuppuswamy B, Al-Kharousi A, Al-Atbi AY, Rizvi S, et al. The effect of change in posture on spirometry in patients with obstructive sleep apnoea syndrome. Sultan Qaboos Univ Med J 2019 Nov;19(4):e310-e315.
- 4. Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep 2007 Jan;30(1):29-34.
- 5. Said EA, Al-Abri MA, Al-Saidi I, Al-Balushi MS, Al-Busaidi JZ, Al-Reesi I, et al. Altered blood cytokines, CD4 T cells, NK and neutrophils in patients with obstructive sleep apnea. Immunol Lett 2017 Oct;190:272-278.
- 6. Martelli V, Carelli E, Tomlinson GA, Orchanian-Cheff A, Kuo KH, Lyons OD, et al. Prevalence of elevated hemoglobin and hematocrit levels in patients with obstructive sleep apnea and the impact of treatment with continuous positive airway pressure: a meta-analysis. Hematology 2022 Dec;27(1):889-901.
- 7. Li N, Li HP, Wang P, Yan YR, Li SQ, Li QY. Nocturnal mean oxygen saturation is associated with secondary polycythemia in young adults with obstructive sleep apnea, especially in men. Nat Sci Sleep 2019 Dec;11:377-386.
- 8. Villafuerte FC, Simonson TS, Bermudez D, León-Velarde F. High-altitude erythrocytosis: mechanisms of adaptive and maladaptive responses. Physiology (Bethesda) 2022 Jul;37(4):0.
- 9. Abdel Hammed MR, Tohamy MA, Boshra SZ, Taha SM, Saleh MF. Red cell distribution width is an inflammatory predictor marker of contrast induced nephropathy in patients undergoing percutaneous coronary intervention. Egypt J Immunol 2023 Jul;30(3):1-12.
- 10. Holik H, Krečak I, Gverić-Krečak V, Vučinić Ljubičić I, Coha B. Higher red blood cell distribution width might differentiate primary from secondary polycythemia: a pilot study. Int J Lab Hematol 2021 Apr;43(2):e68-e71.
- 11. Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009 Jul;114(5):937-951.
- 12. Naresh KN, Medeiros LJ. Introduction to the fifth edition of the World Health Organization classification of tumors of hematopoietic and lymphoid tissues. Mod Pathol 2023 Dec;36(12):100330.
- 13. Rha M-S, Jeong Y, Kim J, Kim C-H, Yoon J-H, Cho H-J. Is obstructive sleep apnea associated with erythrocytosis? A systematic review and meta-analysis. Laryngoscope Investig Otolaryngol 2022 Feb;7(2):627-635.
- 14. Nguyen CD, Holty JC. Does untreated obstructive sleep apnea cause secondary erythrocytosis? Respir Med 2017 Sep;130:27-34.
- 15. Al-Abri M, Al-Hashmi K, Jaju D, Al-Rawas O, Al-Riyami B, Hassan M. Gender difference in relationship of apnoea/hypopnoea index with body mass index and age in the Omani population. Sultan Qaboos Univ Med J 2011 Aug;11(3):363-368.
- 16. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med 2017 Mar;13(3):479-504.
- 17. Arish N, Mackay T, Frangulyan R, Riha RL. Haematocrit level in obesity hypoventilation syndrome: a predictor of mortality? Sleep Breath 2022 Mar;26(1):355-358.