Melioidosis is an infectious disease caused by the soil saprophytic gram-negative bacillus Burkholderia pseudomallei. It is endemic to Southeast Asia and Northern Australia, with increasing reports of imported cases in non-endemic areas such as the Middle East.1 Melioidosis can affect any organ but commonly causes pneumonia, bacteremia, deep-seated abscesses, and skin infections.2 Diabetes mellitus and excessive alcohol consumption are common risk factors for melioidosis.3 Neurological manifestations have been reported in 3–5% of cases.3–5 Neuromelioidosis is a rare central nervous system (CNS) manifestation of melioidosis, with a mortality rate of approximately 25%.3–7 Survivors may experience significant morbidity.5,6
B. pseudomallei is intrinsically resistant to many antibiotics.7,8 Unlike other bacterial infections, melioidosis requires an intensive phase of intravenous (IV) antibiotic treatment followed by a prolonged oral eradication phase to prevent relapse.9,10 Ceftazidime and meropenem are recommended for the intensive phase, while co-trimoxazole or amoxicillin/clavulanic acid combination is used for the eradication phase.
However, for neuromelioidosis, the preferred antibiotics for eradication treatment are meropenem along with oral co-trimoxazole.9,10 The Centers for Disease Control and Prevention (CDC) guidelines recommend an initial intensive phase of high-dose meropenem 2 g intravenously administered every eight hours for 4–8 weeks, followed by eradication therapy with two tablets of co-trimoxazole 160 mg/800 mg (sulfamethoxazole 800 mg with trimethoprim 160 mg) every 12 hours for at least six months.9
We present a patient with disseminated melioidosis with severe neurological involvement who had multiple relapses despite receiving repeated courses of treatment as per the CDC guidelines. Eventually, after an extended period of intensive treatment with meropenem IV, the patient achieved recovery.
Case Report
A 37-year-old man presented to the emergency department with a sudden onset of left hemiplegia and left focal motor seizures. He worked as an agricultural laborer in the paddy fields of Kerala. He had a history of alcohol dependence but was a non-smoker. He has diabetes on irregular treatment with no other significant pre-existing illness or history of any immunosuppressive drug use. There was no history of recent injury or broken skin in the extremities.
The patient appeared emaciated with a body mass index of 19.8 kg/m2. He was febrile and had right knee joint arthritis. An immediate non-contrast-enhanced magnetic resonance imaging (MRI) brain showed areas of patchy diffusion restriction with corresponding low apparent diffusion coefficient values in the right frontoparietal cortex. Surrounding T2 fluid attenuated inversion recovery hyperintensity suggestive of edema was present in the adjacent white matter with extension to the thalamocapsular region [Figure 1].
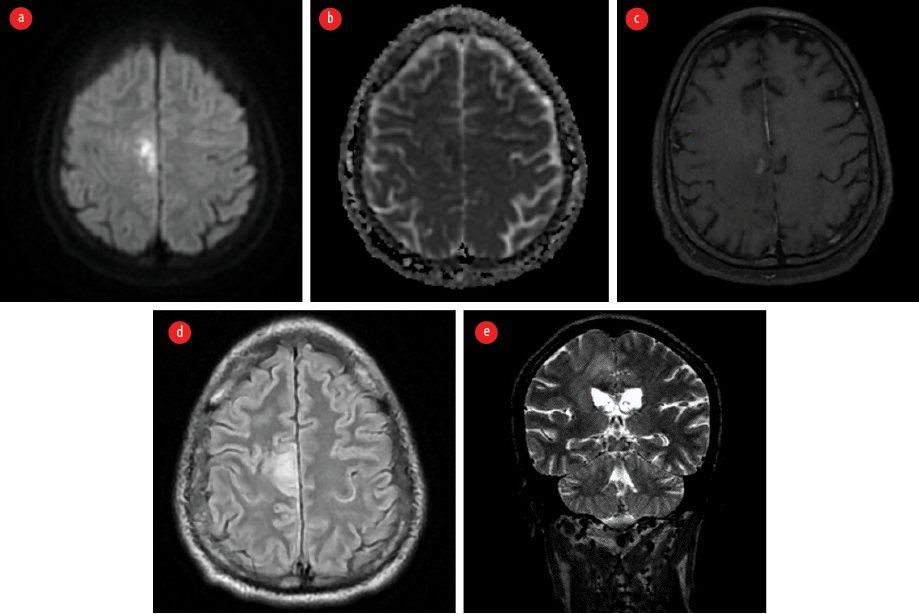 Figure 1: Contrast-enhanced MRI of the brain taken on the first visit. (a) Axial diffusion-weighted image shows areas of diffusion restriction in the right frontoparietal cortex. (b) Corresponding apparent diffusion coefficient map indicates the hypointense areas in the involved region. (c) Axial post contrast T1 image shows patchy contrast enhancement in involved area. (d) Axial T2 fluid attenuated inversion recovery image reveals the surrounding edema. (e) Coronal T2 image shows the edema extending to the thalamocapsular region.
Figure 1: Contrast-enhanced MRI of the brain taken on the first visit. (a) Axial diffusion-weighted image shows areas of diffusion restriction in the right frontoparietal cortex. (b) Corresponding apparent diffusion coefficient map indicates the hypointense areas in the involved region. (c) Axial post contrast T1 image shows patchy contrast enhancement in involved area. (d) Axial T2 fluid attenuated inversion recovery image reveals the surrounding edema. (e) Coronal T2 image shows the edema extending to the thalamocapsular region.
Ultrasonography of the abdomen revealed hepatosplenomegaly, prostatomegaly with abscess formation, and multiple splenic microabscesses [Figure 2]. The culture of his right knee aspirate yielded B. pseudomallei, but the blood culture was negative for the pathogen.
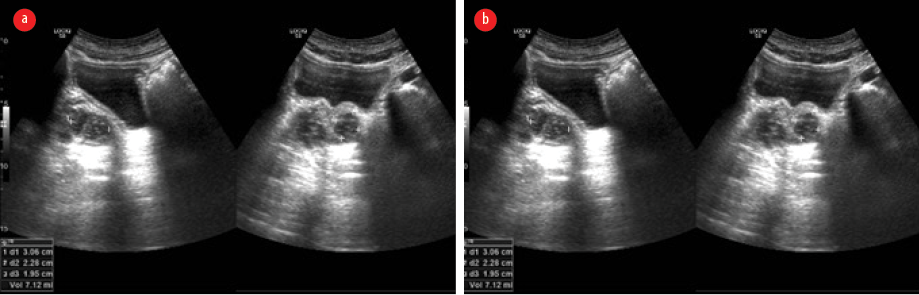 Figure 2: Ultrasonographic images of the abdomen. (a) Hepatosplenomegaly, prostatomegaly with abscess formation. (b) Multiple splenic microabscesses are seen.
Figure 2: Ultrasonographic images of the abdomen. (a) Hepatosplenomegaly, prostatomegaly with abscess formation. (b) Multiple splenic microabscesses are seen.
Based on the above findings, a diagnosis of disseminated melioidosis with CNS involvement was made. The patient was started on intensive treatment as per CDC guidelines — IV meropenem 2 g every eight hours, along with oral co-trimoxazole (800 mg sulfamethoxazole and 160 mg trimethoprim). By the second week, he improved and became afebrile, but left hemiplegia persisted. After four weeks, intensive treatment with IV meropenem was discontinued, and eradication treatment with oral co-trimoxazole was continued. The patient was discharged to a rehabilitation facility.
However, one month later, while still on oral co-trimoxazole, the patient presented with fever, headache, recurrent left focal motor seizures, and eventually fell into a coma, necessitating mechanical ventilation. Repeat contrast-enhanced MRI of the brain revealed irregular, streak-like enhancing areas with surrounding edema, originating in the precentral gyrus of the right frontal lobe and extending to the posterior limb of the internal capsule and the midbrain via the cerebral crus. It was noted to cross the midline at the level of the red nucleus. This irregular enhancement was also noted to involve the superior cerebellar peduncle [Figure 3].
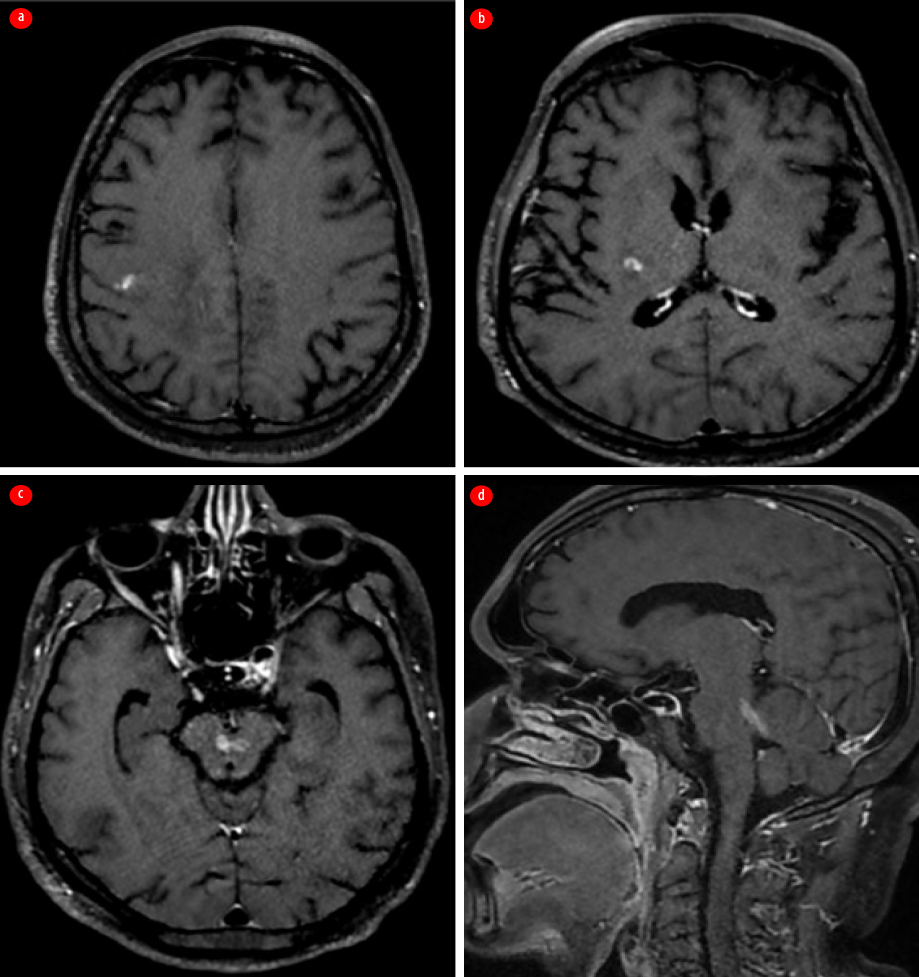 Figure 3: Contrast-enhanced MRI of the brain taken following the first relapse. (a) Axial section shows irregular streak-like enhancing areas starting at the right prefrontal gyrus; (b) Axial section shows extension of enhancement along the right posterior limb of the internal capsule. (c) Axial section shows involvement of the midbrain with crossing of the midline. (d) Sagittal section indicates entry into the cerebellum via the left superior cerebellar peduncle.
Figure 3: Contrast-enhanced MRI of the brain taken following the first relapse. (a) Axial section shows irregular streak-like enhancing areas starting at the right prefrontal gyrus; (b) Axial section shows extension of enhancement along the right posterior limb of the internal capsule. (c) Axial section shows involvement of the midbrain with crossing of the midline. (d) Sagittal section indicates entry into the cerebellum via the left superior cerebellar peduncle.
A blood culture was repeated, but it yielded no growth. Intensive treatment with IV meropenem and oral co-trimoxazole was resumed, once again leading to gradual improvement, resolution of seizures, and extubation after four weeks. Since then, he remained fully conscious but had residual left hemiplegia as his only deficit. After eight weeks, IV meropenem was stopped, and he continued on oral co-trimoxazole.
Two months later, while continuing the eradication treatment, the patient had another relapse and presented with headache and left focal motor seizures. A repeat contrast-enhanced MRI of the brain showed findings similar to the previous one, with additional edema in the ventral aspect of the medulla (more affected on the right side), which extended to the upper cervical cord, affecting the right spinothalamic and left lateral corticospinal tracts [Figure 4].
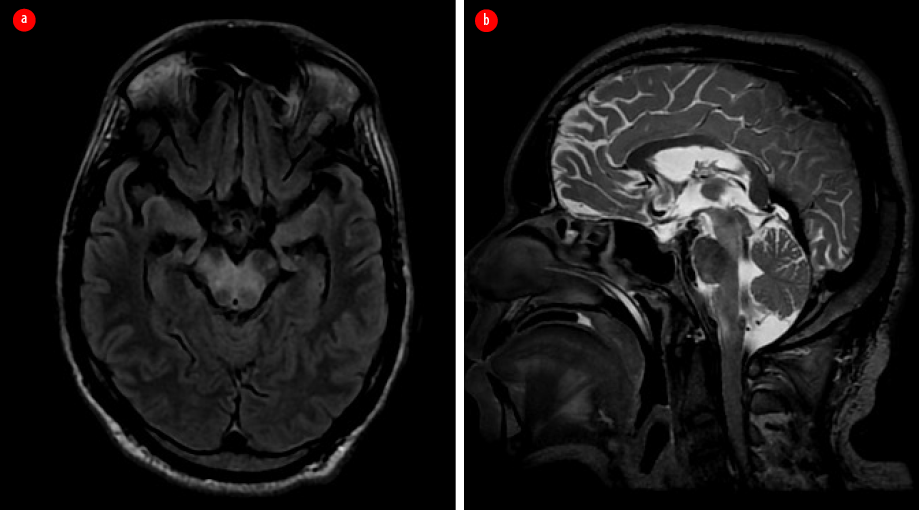 Figure 4: Repeated contrast-enhanced MRI after the second relapse. (a) Axial T2 fluid attenuated inversion recovery image. (b) Sagittal T2-weighted image shows new-onset hyperintensity involving the ventral aspect of pons and medulla.
Figure 4: Repeated contrast-enhanced MRI after the second relapse. (a) Axial T2 fluid attenuated inversion recovery image. (b) Sagittal T2-weighted image shows new-onset hyperintensity involving the ventral aspect of pons and medulla.
The third course of intensive treatment with meropenem IV, along with co-trimoxazole, was initiated, eventually leading to good recovery. Meropenem IV was continued for another eight weeks. Trimoxazole was initiated, leading to the patient’s significant improvement and eventual recovery within four weeks. The third intensive treatment course (a combination of IV meropenem and oral co-trimoxazole) continued for eight weeks, after which IV meropenem was discontinued. Still, oral co-trimoxazole was continued for an additional four months, resulting in the oral eradication phase lasting a total of one year.
Although the patient is unable to return to his previous occupation due to residual left hemiparesis, he remains symptom-free and is on regular outpatient follow-up. Financial constraints prevented a repeat MRI. Informed consent was obtained from the patient.
Discussion
Our patient had culture-proven B. pseudomallei infection with dissemination into the CNS, likely through bacteremia or retrograde axonal transmission along the olfactory and trigeminal nerves.11,12 He also exhibited a stroke-like presentation with diffusion restriction in the right perirolandic regions, possibly by hematogenous dissemination via the middle cerebral artery. Subsequent MRI evaluations revealed dissemination along the descending corticospinal and cerebellar tracts to the brainstem and upper spinal cord.
Neuromelioidosis is often described as an infective tractopathy, and the affinity of B. pseudomallei for white matter tracts, specifically the corticospinal tract, through the formation of multiple microabscesses, is well-documented.3,13 A wide range of imaging presentations has been reported in neuromelioidosis.13–15 Solitary or multiple intracranial microabscesses, frequently accompanied by cerebritis, are commonly observed. They appear on MRI as ring-enhancing lesions with diffusion restriction and low apparent diffusion coefficient values. Extradural/dural disease with overlying calvarial involvement and scalp-based collections, marrow edema with enhancement, and leptomeningeal disease presenting as leptomeningeal thickening and enhancement are also rarely reported.13–15
Despite receiving repeated treatment as per the CDC guidelines,13 our patient suffered multiple clinical relapses. Radiological evidence of white matter dissemination was demonstrated even during proper oral eradication treatment. Currently, meropenem is the preferred drug for neuromelioidosis due to its rapid bactericidal effect, longer post-antibiotic effects, lower toxicity compared to ceftazidime, and lower incidence of treatment failure.16 There have been no reports of primary or acquired resistance to meropenem in B. pseudomallei.8 However, our patient underwent a total of 20 weeks of intensive meropenem treatment to achieve a complete cure.
As a facultative intracellular pathogen, B. pseudomallei can evade the host’s immune response, particularly in immunocompromised patients. The suppressed immune activation associated with diabetes mellitus and alcoholism in our patient may have allowed the bacteria to persist in intracellular locations and disseminate through white matter tracts.17–19 Such patients may require longer periods of intensive-phase antibiotic therapy to avoid relapses, as in the present case. Australian researchers have stressed the importance of the intensive phase treatment, recommending at least eight weeks of IV meropenem and suggesting the possibility of further extension based on disease severity and clinical course.10 Previous studies have recommended an intensive phase for preventing mortality, followed by oral eradication treatment to prevent relapse.20,21 However, our case highlights the need for a more extended intensive therapy period to achieve recovery and prevent relapses, raising questions about the efficacy and necessity of oral eradication treatment.
Conclusion
The patient encountered multiple relapses despite receiving two four-week courses as per CDC-recommended intensive treatment with meropenem. His eventual recovery was achieved through an eight-week-long intensive treatment using the same antibiotic. This case highlights the critical need for prolonged intensive phase treatment with meropenem in cases of protracted neuromelioidosis, which has not been adequately addressed in previous studies. It also raises concerns regarding the effectiveness and necessity of oral eradication therapy. Further research is needed to determine the optimal duration of the intensive phase and assess the necessity of the eradication phase.
Disclosure
The authors declare no conflicts of interest.
references
- 1. Tamtami NA, Khamis F, Al-Jardani A. Imported case of melioidosis in Oman: case report. Oman Med J 2017 Jan;32(1):62-65.
- 2. White NJ. Melioidosis. Lancet 2003 May;361(9370):1715-1722.
- 3. Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 2010 Nov;4(11):e900.
- 4. Kingsley PV, Leader M, Nagodawithana NS, Tipre M, Sathiakumar N. Melioidosis in Malaysia: a review of case reports. PLoS Negl Trop Dis 2016 Dec;10(12):e0005182.
- 5. Currie BJ, Fisher DA, Howard DM, Burrow JN. Neurological melioidosis. Acta Trop 2000 Feb;74(2-3):145-151.
- 6. Wongwandee M, Linasmita P. Central nervous system melioidosis: a systematic review of individual participant data of case reports and case series. PLoS Negl Trop Dis 2019 Apr;13(4):e0007320.
- 7. Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DA, et al. Melioidosis. Nat Rev Dis Primers 2018 Feb;4:17107.
- 8. Schweizer HP. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 2012 Dec;7(12):1389-1399.
- 9. Lipsitz R, Garges S, Aurigemma R, Baccam P, Blaney DD, Cheng AC, et al. Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei infection, 2010. Emerg Infect Dis 2012 Dec;18(12):e2.
- 10. Pitman MC, Luck T, Marshall CS, Anstey NM, Ward L, Currie BJ. Intravenous therapy duration and outcomes in melioidosis: a new treatment paradigm. PLoS Negl Trop Dis 2015 Mar;9(3):e0003586.
- 11. St John JA, Ekberg JA, Dando SJ, Meedeniya AC, Horton RE, Batzloff M, et al. Burkholderia pseudomallei penetrates the brain via destruction of the olfactory and trigeminal nerves: implications for the pathogenesis of neurological melioidosis. mBio 2014 Apr;5(2):e00025-14.
- 12. Liu PJ, Chen YS, Lin HH, Ni WF, Hsieh TH, Chen HT, et al. Induction of mouse melioidosis with meningitis by CD11b+ phagocytic cells harboring intracellular B. pseudomallei as a Trojan horse. PLoS Negl Trop Dis 2013 Aug;7(8):e2363.
- 13. Hsu CC, Singh D, Kwan G, Deuble M, Aquilina C, Korah I, et al. Neuromelioidosis: craniospinal MRI findings in Burkholderia pseudomallei infection. J Neuroimaging 2016;26(1):75-82.
- 14. Mannam P, Arvind VH, Koshy M, Varghese GM, Alexander M, Elizabeth SM. Neuromelioidosis: a single-center experience with emphasis on imaging. Indian J Radiol Imaging 2021 Jan;31(1):57-64.
- 15. Hsu CC, Singh D, Watkins TW, Kwan GN, Skalski M, Hapugoda S, et al. Serial magnetic resonance imaging findings of intracerebral spread of listeria utilising subcortical U-fibres and the extreme capsule. Neuroradiol J 2016 Dec;29(6):425-430.
- 16. Cheng AC, Fisher DA, Anstey NM, Stephens DP, Jacups SP, Currie BJ. Outcomes of patients with melioidosis treated with meropenem. Antimicrob Agents Chemother 2004 May;48(5):1763-1765.
- 17. Willcocks SJ, Denman CC, Atkins HS, Wren BW. Intracellular replication of the well-armed pathogen Burkholderia pseudomallei. Curr Opin Microbiol 2016 Feb;29:94-103.
- 18. Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med 2015 Feb;36(1):111-125.
- 19. Gan YH. Interaction between Burkholderia pseudomallei and the host immune response: sleeping with the enemy? J Infect Dis 2005 Nov;192(10):1845-1850.
- 20. Limmathurotsakul D, Chaowagul W, Chierakul W, Stepniewska K, Maharjan B, Wuthiekanun V, et al. Risk factors for recurrent melioidosis in northeast Thailand. Clin Infect Dis 2006 Oct;43(8):979-986.
- 21. Chaowagul W, Chierakul W, Simpson AJ, Short JM, Stepniewska K, Maharjan B, et al. Open-label randomized trial of oral trimethoprim-sulfamethoxazole, doxycycline, and chloramphenicol compared with trimethoprim-sulfamethoxazole and doxycycline for maintenance therapy of melioidosis. Antimicrob Agents Chemother 2005 Oct;49(10):4020-4025.