Thrombotic thrombocytopenic purpura (TTP) is a rare condition, categorized into two types: congenital (cTTP) and acquired (aTTP). TTP is characterized by a severe deficiency of the enzyme disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS-13). The ADAMTS-13 enzyme cleaves ultra-large von Willebrand molecules and thus controls the thrombosis cascade.1 A second trigger is usually required for this deficiency to manifest clinically. Transforming growth factor beta-1 and interleukin-10 are key regulators of immune homeostasis with anti-tumor effects.2,3 Elevated plasma transforming growth factor beta-1 levels contribute to the progression of TTP, leading to a procoagulant phenotype.4 Additionally, the signal transducer and activator of transcription 3 is essential for efficient interleukin-10-induced TTP expression.5
Health conditions characterized by high blood flow, increased shear forces, and an inflammatory state within tiny capillaries and arterioles can trigger a clinical episode. Potential triggers include infections, pregnancy, malignancy, systemic lupus erythematosus, toxins, and drugs.6 The reported case fatality rate in untreated cases was as high as 90%, while it was 10–20% in patients who receive aggressive treatment.7
With the rise of immunotherapy in cancer treatment, the incidence of immune-related toxicities such as TTP has also increased. For example, ipilimumab, a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blocking antibody, is widely used in cancer treatment.8 This anti-CTLA-4 monoclonal antibody positively affects relapsed acute myeloid leukemia.9 In addition, toll-like receptor 2 expression significantly increased in acute myeloid leukemia patients with mixed fungal and bacterial infections.10 Toll-like receptor 2 ligand enhanced the antitumor efficacy of anti-CTLA-4 by increasing Fcγ receptor IV expression.11
In the UK, the yearly incidence of TTP was 1.2 per million, while in Germany, the annual incidence of aTTP episodes was 2.1 per million. In North America, the yearly incidence of TTP and hemolytic uremic syndrome were reported to be 3.7 and 3.8 cases per million, respectively, with similar numbers from Europe.7 Most studies have reported a predominance of women aged 30–50 years among TTP patients. Children constituted approximately 10% of aTTP cases.7
In the Middle Eastern region, two studies from Egypt reported 22 and 30 cases over 20 years, with an overall mean age of 24 and a median of 46 years. The studies reported female predominance of 65% and 73%, and case-fatality rates of 9% and 13%, respectively.12,13 A subsequent Egyptian study on 31 adult TTP patients found that all had significantly lower levels of ADAMTS-13 compared to healthy controls. Further, no patient had a missense mutation in the enzyme.14 Data from a single center in Jordan showed 21 patients, where 67% were females, 81% had neurological symptoms upon presentation, the mean age was 36, and the mortality rate was 38%.15
In the Gulf Cooperation Council (GCC) countries, TTP epidemiological data was not available except for a 2011 Kuwaiti study that described four cases.7 In 2022, an expert panel reported the annual incidence of aTTP in the UAE, and Saudi Arabia as 1–2 and 5–6 per million persons, respectively.16
It was agreed that more physician awareness about aTTP was needed, along with increased access to rapid testing with ADAMTS-13 enzyme and new TTP treatments.16 Experts from the GCC agreed that there was still a lack of published data on early diagnosis and treatment modalities of aTTP patients.7
This study sought to provide background data on the epidemiology of TTP in the Omani population to overcome the research gap.
Methods
The data for this retrospective cohort study was sourced from the electronic records of patients with aTTP or cTTP diagnoses, between January 2006 and December 2019, seen at two major tertiary centers in Muscat; the Royal Hospital and Sultan Qaboos University Hospital. The medical research ethics committees at both hospitals reviewed and approved the study (SRC# 8/2018; MREC#2022).
Patients of Omani nationality who were at least 12 years old and had one or more documented TTP episodes were selected for the study. Potential participants were excluded if they had incomplete data or were diagnosed with conditions that can mimic TTP, such as severe sepsis, hypertensive crisis, HIV, and microangiopathic anemia in organ transplant patients.
Data gathered pertained to patient demographics, clinical presentation, laboratory results, vital signs, procedures and medications administered, outcomes, and coded diagnoses. Additionally, the blood bank logbook data was used to identify all cases that underwent therapeutic plasma exchange (TPE) in the two hospitals.
A TTP episode was defined as one or more inpatient stays with thrombotic microangiopathy as defined by the International Classification of Diseases,16 and have had one or more TPE procedures during the same inpatient stay, or one or more documented ADAMTS-13 test results of < 10%. A relapse was defined as a new TTP presentation after 30 days of discharge from the initial episode.
The data was analyzed using SPSS Statistics (IBM Corp. Released 2023. IBM SPSS Statistics for Windows, Version 29.0.2.0 Armonk, NY: IBM Corp). Categorial variables were described as frequencies and percentages, and continuous variables as mean (or median) with SD. The total Omani population during the study period ranged from 1.9 to 2.5 million, with an average Omani population of 2.3 million.17,18 The incidence of TTP in Oman was calculated by dividing the number of patients with a newly documented TTP diagnosis during a specific year by the total Omani population during that year, multiplied by one million.
Results
A total of 54 patients met the inclusion criteria. Figure 1 illustrates the number of cases according to the year of diagnosis. Based on the Omani population during the study period, the annual incidence of TTP ranged from 0.5–3.6 cases per million, with an overall average of 1.8 per million. Our adult cohort population had an age of onset of TTP ranging from the 1st –79th year of life. Congenital TTP patients’ symptoms began to manifest in their childhood itself. Interestingly, up to 61% of TTP patients had their first presentation between the third and fifth decade of life. TTP in our study population predominated in females with a female-to-male ratio of 2.2:1.0. An onset of TTP in the postpartum period was observed in six patients [Figure 2]. The following clinical findings were documented during the acute presentation of TTP: fever (63.0%), petechiae/ecchymosis (35.2%), seizures (25.9%), headache (27.8%), confusion (24.1%), stroke (18.5%), and coma 3.7% [Table 1].
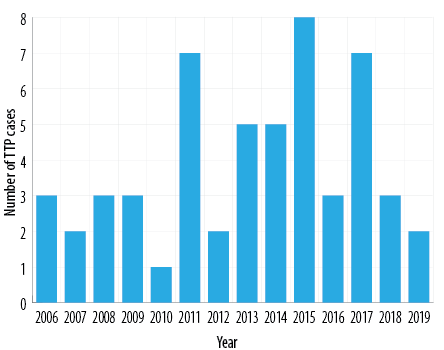 Figure 1: Yearly incidence of reported cases of thrombotic thrombocytopenic purpura (TTP) during 2006–2019 in Omani patients.
Figure 1: Yearly incidence of reported cases of thrombotic thrombocytopenic purpura (TTP) during 2006–2019 in Omani patients.
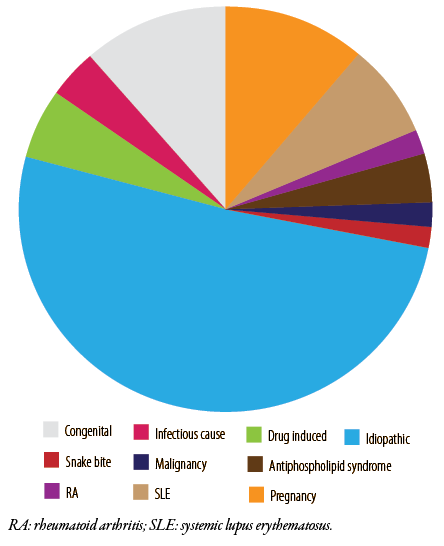 Figure 2: Etiologies of thrombotic thrombocytopenic purpura cases in Oman from 2006–2019.
Figure 2: Etiologies of thrombotic thrombocytopenic purpura cases in Oman from 2006–2019.
Table 1: Demographic characteristics of patients with thrombotic thrombocytopenic purpura during 2006–2019 (N = 54).
|
Sex
|
|
|
|
Female
|
37
|
68.5
|
|
Male
|
17
|
31.5
|
|
Female:male ratio:
|
2.2:1.0
|
|
|
Age at onset, years
|
|
|
|
< 20
|
9
|
16.7
|
|
20–30
|
19
|
35.2
|
|
30–40
|
8
|
14.8
|
|
> 40
|
18
|
33.3
|
|
Governorate
|
|
|
|
Al Batinah
|
19
|
35.2
|
|
Muscat
|
13
|
24.1
|
|
A'Dakhiliyah
|
7
|
13.0
|
|
A'Sharqyah
|
6
|
11.1
|
|
Al Wusta
|
3
|
5.6
|
|
A'Dhahirah
|
1
|
1.9
|
|
Al Buraimi
|
2
|
3.7
|
|
Musandam
|
1
|
1.9
|
As per the patient records, the titer of the ADAMTS-13 enzyme was measured in only 23 (42.8%) patients, among whom 16 (69.6%) were found to have aTTP, indicated by either deficiency in (< 10%) ADAMTS-13 enzyme in six (26.1%), or anti-ADAMTS-13 autoantibodies in 10 (43.5%) patients. Normal enzyme level was found in seven patients. Three patients were found to have cTTP. They were from Al-Wusta governorate. They responded to fresh frozen plasma infusion during relapses without TPE [Table 2].
Table 2: Summary of clinical presentation and laboratory results of Omani patients with thrombotic thrombocytopenic purpura (N = 54).
|
Clinical presentations
|
|
|
|
Fever
|
34
|
63.0
|
|
Petechiae/ecchymosis
|
19
|
35.2
|
|
Seizures
|
14
|
25.9
|
|
High blood pressure
|
14
|
25.9
|
|
Headache
|
15
|
27.8
|
|
Confusion
|
13
|
24.1
|
|
Postpartum period
|
6
|
11.1
|
|
Stroke
|
10
|
18.5
|
|
Coma
|
2
|
3.7
|
|
Underlying malignancy
|
1
|
1.9
|
|
Baseline laboratory results
|
|
ANA titer > 1:60
|
10
|
18.5
|
|
HIV positive (ELISA test)
|
0
|
-
|
|
Deranged renal function (creatinine)
(eGFR6 < 90 mL/kg/min)
|
25
|
46.3
|
|
Deranged coagulation (INR > 1.3)
|
0
|
-
|
|
Hemoglobin, mean (range), g/dL
|
7.5 ± 2.6
(5.0–10.0)
|
|
|
Platelet count, mean (range), /L
|
25.0 × 109
(3.0–120.0 × 109)
|
|
|
ADAMTS-13, n = 23
|
|
|
|
Deficiency (< 10%)
|
6
|
26.1
|
ANA: antinuclear antibodies; eGFR: estimated glomerular filtration rate; INR: international normalized ratio; ADAMTS-13: a disintegrin and metalloproteinase motif 13.
Table 3 lists the treatments administered to the TTP patients. These included steroids (94.4%), TPE (77.8%), rituximab (42.6%), and cyclosporin (18.5%). One cTTP patient with a congenital deficiency of ADAMTS-13 received rituximab. Moreover, cTTP patients with congenital ADAMTS-13 deficiency required fewer plasma exchange sessions (0–7 sessions) and had shorter stays (maximum one week). In comparison, up to 75.0% of patients with acquired ADAMTS-13 deficiency who received rituximab had a more prolonged inpatient stay and required 5–30 TPE sessions [Table 3].
Table 3: Treatments given to Omani patients with thrombotic thrombocytopenic purpura (TTP) (N = 54).
|
Steroids (prednisolone, methylprednisolone, or hydrocortisone)
|
51 (94.4)
|
|
|
Fresh frozen plasma
|
3 (5.5)
|
Congenital TTP
|
|
Cyclosporin
|
10 (18.5)
|
Administered as an adjuvant after rituximab and in post-renal transplant cases
|
|
Rituximab
|
23 (42.6)
|
|
Renal derangement and neurological deficits were common, but most were transient. Of the 25 patients with renal derangement at presentation, nine went on to have stage 2–3 chronic kidney disease. However, all of these patients continued to recover slowly as per their last follow-up visit records. One patient who developed end-stage renal disease remained on regular hemodialysis. This patient, who presented with low hemoglobin during her postpartum period, had severe renal derangement which was managed with > 30 sessions of TPE, prednisolone, rituximab, and cyclosporine. Two of the 10 patients with neurological sequelae continued to have residual weakness, and both had connective tissue disease. Two others were comatose on presentation, from which they recovered completely before discharge. All patients with seizures were administered anti-epileptic medications and remained free of seizures [Table 4].
Table 4: Complications and outcomes among Omani TTP patients (N = 54).
|
Stroke
|
10 (18.5)
|
Plasma exchange and steroids
|
Two patients had a residual neurological deficit on discharge.
|
|
Seizures
|
14 (25.9)
|
Levetiracetam,
plasma exchange, and
steroids
|
All seizures were under control at discharge.
Two patients had connective tissue disease, SLE, and APLS.
One patient developed drug-induced TTP.
Two patients passed away with TTP.
|
|
Coma
|
2 (3.7)
|
Plasma exchange and steroids
|
Both recovered.
|
|
CKD
|
9 (16.6)
|
Plasma exchange, steroids, and hemodialysis
|
Half of the cases developed CKD stages 2–3. All recovered as per the last available record.
Three patients had connective tissue disease, SLE, RA, and APLS.
One patient had a snake bite.
|
TTP: thrombotic thrombocytopenic purpura; CKD: chronic kidney disease; SLE: systemic lupus erythematosus; APLS: antiphospholipid syndrome; RA: rheumatoid arthritis; BMT: bone marrow transplant; GvHD: graft-versus-host disease.
TTP relapse was recorded in seven (13.0%) patients within 1–7 years (mean = 3.5 ± 2.8) of the initial episode. This study’s case fatality rates were five (9.3%) at 30 days and seven (13.0%) at 90 days. The seven patients who died were aged 13–87 years (mean = 46.0 ± 30.1).
Discussion
This study evaluated the TTP incidence, treatment modalities, and clinical outcomes among Omani patients > 13 years from 2006 to 2019. The incidence of TTP in Oman was found to be 1.8 per million per year, lower than the global rate of 3 per million as well as the GCC incidence of 3–6 per million. This raises the possibility that TTP is underdiagnosed in Oman.
TTP onset in the postpartum period was observed in six of our female patients. Many case series reported pregnancy as a precipitating factor for acute episodes of TTP with hemolytic uremic syndrome.13 This association has been explained as being due to the increased concentration of procoagulant factors, decreased activity of ADAMTS-13, reduced fibrinolytic activity, and loss of endothelial cells. These changes increase severity during the pregnancy, peaking at delivery and the immediate postpartum period.19,20
Although most of our patients received the appropriate conventional therapy, mortality and stroke were high at 13.0% and 18.5%, respectively. This indicates late diagnosis, raising the need for increased physician awareness to facilitate early diagnosis, prompt treatment, and adoption of novel therapies to improve outcomes. As in the other GCC countries the relapse rate observed in this study, at 13.0%, was much lower than the 30–50% range reported globally.7,21–23 Moreover, relapses were more common among our cTTP patients than those with aTTP.
Recent reports described the survival rates of over 95% in patients receiving caplacizumab, the latest recommended standard of care, along with TPE, immunosuppression, and novel monoclonal therapy. Caplacizumab was approved for TTP treatment in combination with steroids and TPE in 2018 in Europe, 2019 in the USA,16 and later in the
GCC countries, including Oman. Therefore, our findings reflected the outcome of only more conventional therapies.
The consequences of TTP can last beyond the acute phase, as indicated by several studies, including one from the UK which found that patients who presented with acute TTP went on to have impairments in quality of life, cognitive function, psychological well-being, and physical functioning.16 Similarly, our study showed that TTP was associated with significant comorbidities, many of which were precipitated by TTP, such as kidney dysfunction and neurological symptoms. Additionally, there was regional clustering of cTTP in Al Wusta governorate despite its tiny population. This indicates the need to enhance genetic counseling in this rural and traditional governorate.
The present study strengthens the evidence that research and treatment gaps exist for TTP in Oman. However, it had the usual limitations of retrospective retrieval of data recorded for medical purposes rather than research. In addition, the assessment of ADAMTS-13 was conducted on fewer than half of our patients, as the samples had to be outsourced abroad for testing.
Conclusion
This study has provided TTP incidence, treatment modalities, and clinical outcomes in the Omani population. According to our results, Oman has a lower incidence of TTP than other GCC countries as well as globally. This suggests the possibility of TTP being underdiagnosed in Oman. The high case fatality rate and morbidity in Omani patients with TTP, despite treatment with TPE and immunosuppression, highlightes the need for easy access to ADAMTS-13 testing in Oman and increasing physician awareness to enable early diagnosis and treatment of this rare but life-threatening disease.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
references
- 1. Saha M, McDaniel JK, Zheng XL. Thrombotic thrombocytopenic purpura: pathogenesis, diagnosis and potential novel therapeutics. J Thromb Haemost 2017 Oct;15(10):1889-1900.
- 2. Abdel Hammed MR, Ahmed YA, Adam EN, Bakry R, Elnaggar MG. sVCAM-1, and TGFβ1 in chronic phase, chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Egypt J Immunol 2022 Oct;29(4):163-173.
- 3. Mohammed D, Khallaf S, El-Naggar M, Abdel-Hameed M, Bakry R. Interleukin-10: a potential prognostic marker in patients with newly diagnosed multiple myeloma. Resum Oncol 2021;17(1):38-41.
- 4. Zauli G, Gugliotta L, Catani L, Vianelli N, Borgatti P, Belmonte MM, et al. Increased serum levels of transforming growth factor beta-1 in patients affected by thrombotic thrombocytopenic purpura (TTP): its implications on bone marrow haematopoiesis. Br J Haematol 1993 Jul;84(3):381-386.
- 5. Gaba A, Grivennikov SI, Do MV, Stumpo DJ, Blackshear PJ, Karin M. Cutting edge: IL-10–mediated tristetraprolin induction is part of a feedback loop that controls macrophage STAT3 activation and cytokine production. J Immunol 2012 Sep;189(5):2089-2093.
- 6. George JN, Al-Nouri ZL. Diagnostic and therapeutic challenges in the thrombotic thrombocytopenic purpura and hemolytic uremic syndromes. Hematology Am Soc Hematol Educ Program 2012;2012(1):604-609.
- 7. Al-Khabori M, Alsayegh F, Al Yaseen H, Hussien S, Lal A, Al Rasheed M, Al Bader M, et al. The challenges in diagnosis and management of acquired thrombotic thrombocytopenic purpura: a consensus report from three gulf countries. Oman Med J 2022;37(4):e407.
- 8. King J, de la Cruz J, Lutzky J. Ipilimumab-induced thrombotic thrombocytopenic purpura (TTP). J Immunother Cancer 2017 Mar;5:19.
- 9. Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al; Leukemia and Lymphoma Society Blood Cancer Research Partnership. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med 2016 Jul;375(2):143-153.
- 10. Abdel Hammed MR, Elgendy SG, El-Mokhtar MA, Sayed D, Mansour SM, Darwish AM. T-lymphocytes expression of toll-like receptors 2 and 4 in acute myeloid leukemia patients with invasive fungal infections. Mediterr J Hematol Infect Dis 2022 Mar;14(1):e2022022.
- 11. Sharma N, Vacher J, Allison JP. TLR1/2 ligand enhances antitumor efficacy of CTLA-4 blockade by increasing intratumoral Treg depletion. Proc Natl Acad Sci U S A 2019 May;116(21):10453-10462.
- 12. Kueh YK. Adult idiopathic thrombocytopenic purpura (ITP)–a prospective tracking of its natural history. Singapore Med J 1995 Aug;36(4):367-370.
- 13. El-Husseiny NM, Goubran H, Fahmy HM, Tawfik NM, Moustafa H, Amin SN, et al. Outcome and relapse risks of thrombotic thrombocytopaenic purpura: an Egyptian experience. Postgrad Med J 2012 Aug;88(1042):433-436.
- 14. El Sissy MH, El Hafez AA, El Sissy AH. Low incidence of ADAMTS13 missense mutation R1060W in adult Egyptian patients with thrombotic thrombocytopenic purpura. Acta Haematol 2014;132(1):30-35.
- 15. Obeidat M, Al-Swailmeen A, Bawa’neh A, Arabeiat A, AbuKamar A, Almomani A, et al. Thrombotic thrombocytopenic purpura: a single-center experience in Jordan. Natl J Med Res 2020;10(1):16-20.
- 16. Hasan AY, Al Mehairi A, Aldarweesh M, Damlaj M, El Tayeb K, Hussain S, et al. Burden of acquired thrombotic thrombocytopenic purpura: KSA and UAE expert consensus for improved disease management. Journal of Applied Hematology 2022 Jul;13(3):145-153.
- 17. Arabic People. 2019 [cited 2023 July 29]. Available from: http://arabic.people.com.cn/n3/2019/0611/c31662-9586430.html.
- 18. National Centre for Statistics and Information. NCSI Oman [cited 2023 July 23]. Available from: http://ncsi.gov.om.
- 19. Yoshii Y, Fujimura Y, Bennett CL, Isonishi A, Kurumatani N, Matsumoto M. Implementation of a rapid assay of ADAMTS13 activity was associated with improved 30-day survival rate in patients with acquired primary thrombotic thrombocytopenic purpura who received platelet transfusions. Transfusion 2017 Aug;57(8):2045-2053.
- 20. George JN. The association of pregnancy with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Curr Opin Hematol 2003 Sep;10(5):339-344.
- 21. Abou-Ismail MY, Arafah Y, Fu P, Cao S, Schmaier AH, Nayak L. Outcomes of immune thrombotic thrombocytopenic purpura (iTTP) with upfront cyclophosphamide vs. rituximab. Front Med (Lausanne) 2020 Oct;7:588526.
- 22. Osborn JD, Rodgers GM. Update on thrombotic thrombocytopenic purpura. Clin Adv Hematol Oncol 2011 Jul;9(7):531-536.
- 23. Maloney N, Martin I, Szczepiorkowski ZM, Dunbar NM. Therapeutic plasma exchange for thrombotic thrombocytopenic purpura with refractory thrombocytopenia. J Clin Apher 2018 Jun;33(3):436-438.