Q fever is a worldwide zoonosis caused by the obligate intracellular gram-negative coccobacillus Coxiella burnetii, named for the uncertainty of its etiology when first discovered. The main reservoirs in the environment are farm animals, and the primary transmission route is through contact with infected animals or the consumption of unpasteurized dairy products.1 Hard ticks may also play a role in transmission, though not a major one.2 The disease can manifest in acute or chronic forms, but diagnosis is often challenging. The prevalence of Q fever in Oman is unknown. Over a 15-year period (from January 2009 to November 2023) at Sultan Qaboos University Hospital, we treated a number of patients for whom Q fever was considered a possible diagnosis, based primarily on their clinical presentations and supported by available laboratory investigations (qualitative and quantitative serology and nucleic acid amplification test (NAAT)). Here, we share our experience shedding some light on the difficulties in reaching a diagnosis. Characteristics and the sequential serology result of patients are shown in Tables 1 and 2, respectively.
Case one
A 27-year-old man referred from Salalah for splenomegaly and bicytopenia developed jaundice and jerky movements during his admission. He was referred to the infectious diseases team for possible infectious etiology. Q fever serology was positive for immunoglobulin (Ig) G antibodies to phase I antigen (anti-IgG I), and his echocardiogram (ECHO) showed thickening of mitral and aortic valve leaflets without a clear mass. He was commenced on doxycycline and hydroxychloroquine (HCQ). One month later, he was asymptomatic with improved blood parameters and referred back to Salalah for completion of treatment.
Case two
A 59-year-old man presented with a one-week history of fever up to 39 oC, associated with dry cough, reduced appetite, and headache after returning from a five-week visit to Denmark. He received Tazocin for Achromobacter bacteremia. His Q fever serology was positive for anti-IgG I and IgG II. One week after starting doxycycline, he was still febrile, and his anti-citrullinated peptide antibody (ACPA) and rheumatoid factor (RF) were positive. However, he was totally asymptomatic after three weeks, with normal ACPA and RF.
Table 1: Characteristics of 17 patients treated for Q fever.
|
M/27
|
2009
|
Bicytopenia, splenomegaly, jaundice, no fever
|
Salalah
|
NK
|
4.7
|
ALP: 340
Bilirubin: 19
|
178/112
|
Sero qlt
|
|
M/59
|
2011
|
3–4 weeks of fever, dry cough, syncope, headaches, reduced appetite
|
Denmark, Muscat
|
Dog
|
10.4
|
ALT/AST: 2–3 × normal
|
122/65
|
Sero qlt, qnt
|
|
M/35
|
2011
|
5 days of fever, chills, sweating, then 5 days of fever, bilateral knee pain, jaundice, splenomegaly, Achilles tendon pain, mouth ulcers one month before
|
Muscat
|
No
|
4
|
ALT/AST: 2 × normal
|
124/69
|
Sero qlt
|
|
F/41
|
2011
|
2–3 weeks of fever, SOB, cough, chest pain, palps, memory impairment, joint swelling and pain, Flu A
|
A'Sharqiyah
|
NK
|
23.9
|
ALT/AST: 2 × normal
|
98/48
|
Sero qlt
|
|
M/38
|
2013
|
1 month unwell, dizzy, dark urine, jaundice, bloody stools, 3 days of lower abdominal pain, oliguria, hepatitis, 10 kg weight loss in 1 month
|
A'Sharqiyah
|
NK
|
15.7
|
AST: 3 × normal, bilirubin: 240
|
87/34
|
Sero qlt
|
|
F/48
|
2013
|
1 month fever, latent TB 9 months prior, a new murmur in 2015
|
Muscat
|
No
|
7.9
|
Normal
|
73/95
|
Sero qlt
|
|
F/13
|
2013
|
11 days of fever, cough, anorexia, night sweats
|
Al Batinah
|
NK
|
3.3
|
ATL/AST: 1.5 × normal
|
54/41
|
Sero qlt
|
|
M/36
|
2014
|
4 weeks of fever, profuse sweating, 7 kg weight loss, arthralgia 10 days after returning from Thailand
|
Al Batinah
|
Goats
|
7.8
|
Normal
|
75/67
|
Sero qlt
|
|
M/37
|
2014
|
3 months of intermittent fever. TTE showed possible vegetation on mitral valve
|
A'Sharqiyah
|
Yes
|
9.8
|
ALT/AST: marginal increase
|
50
|
Sero qlt
|
|
F/38
|
2015
|
1 month of ever with generalised pain, rigors, sweating after returning from India
|
Al Batinah
|
NK
|
3.2
|
ALT/AST: 3 × normal
|
2/33
|
Sero qlt,
|
|
M/46
|
2016
|
3 months FUO, polyarthritis, anemia, lymphopenia, thrombocytopenia, purpuric rash
|
A'Dhahirah
|
Yes
|
6.4
|
Normal
|
336/93
|
Sero qlt
|
|
F/52
|
2016
|
Fever, hepatitis
|
A'Dhahirah
|
NK
|
3.2
|
ALT/AST: normal, bilirubin: 320
|
14/39
|
Sero qlt,
|
|
F/38
|
2018
|
Infective endocarditis
|
A'Sharqiyah
|
NK
|
29.2
|
Normal
|
83/5
|
Sero qlt, qnt
|
|
M/28
|
2020
|
8 days of fever, chills, headaches, jaundice
|
Muscat
|
Yes
|
6.3
|
ALT: 10 × normal, bilirubin: 79
|
61/NK
|
Sero qlt, PCR
|
|
M/38
|
2021
|
2 months of fever sweating, 6 kg, weight loss
|
A'Dakhiliyah
|
NK
|
5.3
|
Normal
|
13/NK
|
Sero qlt, qnt, PCR
|
|
M/38
|
2021
|
1 week of left-sided weakness, headache, nausea, 1 day dizziness, slurred speech, LOC
|
A'Dakhiliyah
|
Livestock
|
2.6
|
ALT/AST: 2–3 × normal
|
104/NK
|
Sero qlt, qnt
|
WBC: white blood cell; LFT: liver function test; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; NK: not known; ALP: alkaline phosphatase;Sero: serology; qlt: qualitative; ALT: alanine transaminase; AST: aspartate transaminase; qnt: quantitative; SOB: shortness of breath; TB: tuberculosis; TTE: transthoracic echocardiogram; FUO: fever of unknown origin; PCR: polymerase chain reaction; LOC: loss of consciousness.
Table 2: Sequential serology results of patients treated for Q fever with interpretation.
|
Case 1
|
Possible acute infection
|
|
|
|
|
|
|
D0
|
D18
|
D96
|
|
|
|
|
|
|
IgM II
|
+
|
+
|
-
|
|
|
|
|
|
|
IgA I
|
-
|
-
|
-
|
|
|
|
|
|
|
IgG I
|
+
|
+
|
+
|
|
|
|
|
|
|
Case 2
|
Acute infection
|
|
|
|
|
D0
|
D35
|
D105
|
Yr3
|
Yr6
|
|
|
|
|
IgM II
|
+
|
+
|
+
|
-
|
-
|
|
|
|
|
IgG II
|
+
|
+
|
+
|
+
|
eqv
|
|
|
|
|
IgA I
|
+
|
+
|
+
|
-
|
-
|
|
|
|
|
IgG I
|
+
|
+
|
-
|
+
|
+
|
|
|
|
|
Biomnis
|
D35
|
D60
|
|
|
|
|
|
|
|
IgM II
|
+
|
+
|
|
|
|
|
|
|
|
IgM I
|
Weak +
|
Weak +
|
|
|
|
|
|
|
|
IgG II
|
512
|
256
|
|
|
|
|
|
|
|
IgG I
|
< 64
|
< 64
|
|
|
|
|
|
|
|
Case 3
|
Possible acute infection
|
|
|
|
|
|
|
|
D0
|
D110
|
|
|
|
|
|
|
|
IgM II
|
+
|
+
|
|
|
|
|
|
|
|
IgG II
|
+
|
+
|
|
|
|
|
|
|
|
IgA I
|
-
|
-
|
|
|
|
|
|
|
|
IgG I
|
-
|
-
|
|
|
|
|
|
|
|
Case 4
|
Possible acute infection
|
|
|
|
|
|
|
|
D0
|
D80
|
|
|
|
|
|
|
|
IgM II
|
+
|
+
|
|
|
|
|
|
|
|
IgG II
|
+
|
|
|
|
|
|
|
|
|
IgA I
|
Weak +
|
|
|
|
|
|
|
|
|
IgG I
|
-
|
-
|
|
|
|
|
|
|
|
Case 5
|
Possible past infection
|
|
|
|
|
|
|
|
D0
|
D10
|
|
|
|
|
|
|
|
IgM II
|
eqv
|
eqv
|
|
|
|
|
|
|
|
IgG II
|
eqv
|
-
|
|
|
|
|
|
|
|
IgA I
|
+
|
+
|
|
|
|
|
|
|
|
IgG I
|
+
|
+
|
|
|
|
|
|
|
|
Case 6
|
Possible acute infection
|
|
|
|
|
|
|
|
D0
|
D32
|
|
|
|
|
|
|
|
IgM II
|
+
|
eqv
|
|
|
|
|
|
|
|
IgG II
|
+
|
+
|
|
|
|
|
|
|
|
IgA I
|
-
|
-
|
|
|
|
|
|
|
|
IgG I
|
-
|
-
|
|
|
|
|
|
|
|
Case 7
|
Possible acute infection
|
|
|
|
|
|
D0
|
D30
|
D85
|
Y2
|
|
|
|
|
|
IgM II
|
-
|
+
|
Weak +
|
-
|
|
|
|
|
|
IgG II
|
-
|
+
|
+
|
+
|
|
|
|
|
|
IgA I
|
-
|
-
|
-
|
-
|
|
|
|
|
|
IgG I
|
-
|
-
|
-
|
+
|
|
|
|
|
|
Case 8
|
Possible acute of non-specific reaction
|
|
|
|
|
|
|
D0
|
D35
|
|
|
|
|
|
|
|
IgM II
|
+
|
-
|
|
|
|
|
|
|
|
IgG II
|
eqv
|
eqv
|
|
|
|
|
|
|
|
IgA I
|
-
|
-
|
|
|
|
|
|
|
|
IgG I
|
-
|
-
|
|
|
|
|
|
|
|
Case 9
|
Possible past infection
|
|
|
|
|
|
|
D0
|
D15
|
D45
|
|
|
|
|
|
|
IgM II
|
-
|
-
|
-
|
|
|
|
|
|
|
IgG II
|
-
|
eqv
|
-
|
|
|
|
|
|
|
IgA I
|
-
|
-
|
-
|
|
|
|
|
|
|
IgG I
|
+
|
+
|
+
|
|
|
|
|
|
|
Case 10
|
Possible chronic infection
|
|
D0
|
D35
|
D270
|
D360
|
D430
|
D450
|
Yr3
|
Yr4
|
|
IgM II
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
IgG II
|
+
|
+
|
+
|
+
|
-
|
+
|
+
|
+
|
|
IgA I
|
+
|
+
|
+
|
+
|
+
|
+
|
+
|
+
|
|
IgG I
|
+
|
eqv
|
+
|
eqv
|
+
|
+
|
eqv
|
+
|
|
Case 11
|
|
|
|
|
|
|
|
|
|
D0
|
D80
|
|
|
|
|
|
|
|
IgM II
|
eqv
|
-
|
|
|
|
|
|
|
|
IgG II
|
+
|
+
|
|
|
|
|
|
|
|
IgA I
|
-
|
-
|
|
|
|
|
|
|
|
IgG I
|
+
|
+
|
|
|
|
|
|
|
|
Case 12
|
Possible chronic infection
|
|
|
|
|
|
|
D0
|
D350
|
D480
|
|
|
|
|
|
|
IgM II
|
-
|
-
|
-
|
|
|
|
|
|
|
IgG II
|
eqv
|
+
|
+
|
|
|
|
|
|
|
IgA I
|
-
|
+
|
+
|
|
|
|
|
|
|
IgG I
|
-
|
+
|
+
|
|
|
|
|
|
|
Case 13
|
Chronic infection
|
|
|
|
|
|
|
D0
|
D210
|
D450
|
|
|
|
|
|
|
IgM II
|
-
|
-
|
|
|
|
|
|
|
|
IgG II
|
+
|
2048
|
1024
|
|
|
|
|
|
|
IgA I
|
+
|
+
|
+
|
|
|
|
|
|
|
IgG I
|
+
|
1024
|
256
|
|
|
|
|
|
|
Case 14
|
Possible acute infection
|
|
|
|
|
|
|
|
D0
|
D50
|
|
|
|
|
|
|
|
IgM II
|
+
|
+
|
|
|
|
|
|
|
|
IgG II
|
-
|
+
|
|
|
|
|
|
|
|
IgA I
|
-
|
-
|
|
|
|
|
|
|
|
IgG I
|
-
|
-
|
|
|
|
|
|
|
|
Case 15
|
Resolving infection
|
|
|
|
|
|
D0
|
D150
|
D330
|
D630
|
|
|
|
|
|
IgM II
|
+
|
-
|
+
|
-
|
|
|
|
|
|
IgG II
|
+
|
-
|
+
|
+
|
|
|
|
|
|
IgA I
|
-
|
-
|
+
|
+
|
|
|
|
|
|
IgG I
|
-
|
+
|
+
|
+
|
|
|
|
|
|
D0 Lyon France
|
D180 Lyon France
|
D480 Mayo USA
|
|
|
|
|
|
|
IgM II
|
-
|
-
|
< 1:16
|
|
|
|
|
|
|
IgG II
|
2048
|
2048
|
1:128
|
|
|
|
|
|
|
IgA I
|
-
|
-
|
< 1:16
|
|
|
|
|
|
|
IgG I
|
1024
|
1024
|
1:256
|
|
|
|
|
|
|
Case 16
|
Chronic infection
|
|
|
|
D0
|
D24
|
D130
|
D340
|
D490
|
D730
|
|
|
|
IgM II
|
+
|
+
|
+
|
+
|
-
|
-
|
|
|
|
IgG II
|
-
|
+
|
+
|
+
|
+
|
+
|
|
|
|
IgA I
|
-
|
-
|
-
|
+
|
+
|
eqv
|
|
|
|
IgG I
|
-
|
-
|
+
|
+
|
+
|
+
|
|
|
|
Case 17
|
Chronic infection
|
|
|
|
|
|
D130
|
D340
|
D490
|
D580
|
|
|
|
|
|
IgM II
|
+
|
-
|
< 1:16
|
< 1:16
|
|
|
|
|
|
IgG II
|
2048
|
256
|
1:128
|
1:64
|
|
|
|
|
|
IgA I
|
+
|
-
|
< 1:16
|
< 1:16
|
|
|
|
|
|
Ig GI
|
1024
|
256
|
1:128
|
1:256
|
|
|
|
|
|
D0
|
D8
|
D150
|
|
|
|
|
|
|
IgM II
|
+
|
eqv
|
-
|
|
|
|
|
|
|
IgG II
|
+
|
+
|
+
|
|
|
|
|
|
|
IgA I
|
+
|
+
|
+
|
|
|
|
|
|
|
IgG I
|
+
|
+
|
+
|
|
|
|
|
|
|
D14
|
|
|
|
|
|
|
|
|
IgM II
|
-
|
|
|
|
|
|
|
|
|
IgG II
|
> 2048
|
|
|
|
|
|
|
|
|
IgM I
|
-
|
|
|
|
|
|
|
|
D: day; Yr: year; Ig: immunoglobulin; Eqv: equivocal.
Quantitative serology is mentioned when available. Note that IgA was not measured in all tests. IgG II in case 1 was not reported, and repeat testing was not done at standard intervals. Interpretation of the serology clearly illustrates the difficulty in making the diagnosis and distinguishing the acute from the chronic form in the absence of quantitative serology.
Case three
A 35-year-old man presented with a two-week history of fever (40 oC) with chills and rigors, dry eyes, jaundice, and splenomegaly. His Q fever serology was positive for phase II (IgM and Ig) and phase I (IgA and IgG), as well as RF and ACPA. He was started on doxycycline for two weeks, after which he defervesced with a decrease in his inflammatory markers. However, his eye dryness persisted, and he developed pain in his right Achilles tendon and heel. After 1.5 months, he was asymptomatic, and his RF and ACPA were normalized.
Case four
A 41-year-old woman presented with a two-to-three-week history of fever, dyspnea, cough, chest pain, and hand minor joint arthritis, followed by a rash. She required intubation for acute respiratory distress syndrome due to influenza A infection. A computed tomography (CT) brain scan suggested vasculitis, for which she was started on prednisolone 40 mg. Her ECHO showed pericardial effusion, and Q fever serology was positive for IgG II. She was started on doxycycline with HCQ, but after three months of doxycycline alone (HCQ stopped due to intolerance), she was asymptomatic. Three months after stopping doxycycline and tapering steroids, her arthritis recurred, and she was diagnosed with seronegative RA and fibromyalgia.
Case five
A 38-year-old man presented with a one-month of feeling unwell, jaundice, 10 kg weight loss, lower abdominal pain, and per rectal bleeding. He had hepatic and renal impairment with hypotension. He had used an unknown herbal medication after developing jaundice. Tests for Leptospira, dengue, and Crimean-Congo hemorrhagic fever were all negative, as well as his blood cultures and tests for hemophagocytic lymphohistiocytosis. Doxycycline was started based on his equivocal IgM II positive IgG I and as a good antibiotic choice for many infections.
Case six
A 48-year-old woman presented with a one-month history of fever with chills and rigors, weight loss (2 kg), dysuria, and flank pain. Her CT abdomen showed sacroiliitis. She also had a one-year history of knee pain labeled as osteoarthritis. Based on her Q fever serology results (positive for phase II IgM and IgG), she was started on doxycycline and HCQ but continued only on doxycycline due to intolerance to HCQ. Five weeks later, she was completely asymptomatic with normalization of her inflammatory markers.
Case seven
A 13-year-old girl presented with a 10-day history of fever, night sweats, cough, and body aches. Her CT abdomen showed mesenteric lymphadenitis and sacroiliitis. Her tests for tuberculosis were negative, and Q fever serology suggested acute Q fever. Six weeks after starting doxycycline, she was afebrile with resolution of her lymphadenopathy. However, she had a recurrence of her knee pains along with mouth ulcers and was diagnosed with Behçet’s disease. Later, she was found to be heterozygous for the MC4R gene, which is linked to obesity.
Case eight
A 36-year-old man presented with eight weeks of fever (40 oC) with chills and profuse night sweating, decreased appetite, and 7 kg weight loss. Q fever IgM II was positive, and he was started on doxycycline. Two weeks later, he was afebrile but developed new pains in his knee and ankle, despite dropping inflammatory markers. After six weeks, he was afebrile and asymptomatic.
Case nine
A 37-year-old man presented with 12 days of fever reaching 40 oC, peaking at night and afternoon, associated with headache and one month of intermittent right iliac fossa pain associated with reduced appetite but no weight loss. His ECHO showed a suspicious mass, and Q fever serology was positive for IgG I. He was commenced on doxycycline and HCQ and planned for transesophageal ECHO, but he defaulted.
Case 10
A 38-year-old woman with a known case of systemic lupus erythematosus (SLE) with good adherence to medication, presented with a one-month history of high fever starting one week after returning from India, associated with sweating and rigors. She also had body aches, swelling on the dorsum of her hand, sore throat, and a productive cough. CT chest showed pericardial effusion. She was parainfluenza 3 positive, and her Q fever serology showed positive IgG I and II. She was commenced on doxycycline and HCQ, planned for one year. Her fever resolved, as did her pericardial effusion with treatment for Q fever.
Case 11
A 46-year-old man presented with a one-month history of fever with rigors and sweating, polyarthritis (shoulders, small joints of hands, knees, and elbows), and morning stiffness. His Q fever serology was positive for IgG I and II. Two weeks after commencing doxycycline, his joint pain improved by 80% and his fever resolved. However, two weeks later, he again had a fever, arthralgia, and developed non-pruritic purpuric rash, although his C-reactive protein dropped from 147 to 1. His RF and ACPA were negative, and he was considered as having possible seronegative rheumatoid arthritis/adult-onset Still’s disease. He is still under follow-up by the rheumatology unit.
Case 12
A 52-year-old woman presented with jaundice and transaminitis followed by fever and neutropenia. Her bone marrow biopsy showed histiocytes and intracellular organisms with inclusion bodies. Infectious etiology was suggestive of Q fever by serology, but polymerase chain reaction (PCR) was negative. Two weeks after starting doxycycline, she defervesced with improvement in her bilirubin but had a slight increase in transaminases and developed a new maculopapular rash. She was treated with doxycycline for four months and followed up by hepatologists for autoimmune hepatitis.
Case 13
A 38-year-old woman presented with a two-month history of fever (39 oC), 7 kg weight loss, and a productive cough with yellow sputum. She had been treated at her regional hospital with courses of antibiotics without improvement. She also reported a month-long history of pain and swelling in her lower back. Her medical history included SLE and antiphospholipid syndrome, for which she was on mycophenolate and HCQ. A transthoracic ECHO showed a highly mobile mass on the anterior leaflet of the mitral valve [Figure 1]. Four sets of blood cultures were negative. Q fever serology was positive for IgG I and II, and she was commenced on doxycycline with an increase in the HCQ dose to three times per day. Repeat ECHOs in 2019 and 2021 showed a resolution of the vegetation [Figure 2].
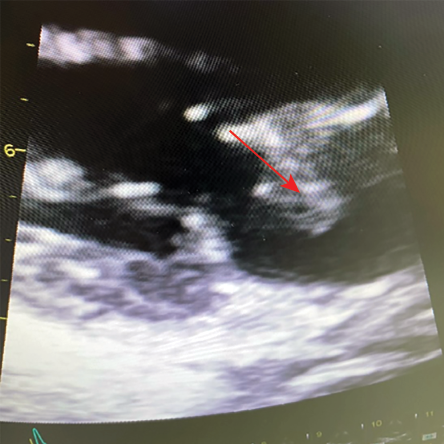 Figure 1: ECHO showing a mass on the anterior leaflet of mitral valve.
Figure 1: ECHO showing a mass on the anterior leaflet of mitral valve.
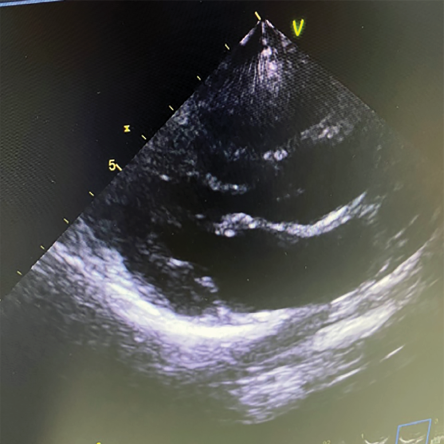 Figure 2: Resolution of the mass after 1.5 years of treatment
Figure 2: Resolution of the mass after 1.5 years of treatment
Case 14
A 28-year-old man presented with a one-week history of fever (39 oC) with shivering, nausea, jaundice, dysuria, decreased appetite, and dark urine. His bilirubin was 79 µmol/L. He had swum in a falaj (irrigation water aqueducts) in the week before his illness. Leptospira serology was negative, but Q fever serology was positive for IgM II and IgG II. He was started on doxycycline, and two weeks later, he defervesced with improvement in his liver function test.
Case 15
A 38-year-old man presented with two months of fever (39.5 oC) with profuse sweating, reduced appetite, and weight loss of 6 kg. Q fever PCR from blood was positive, and he was commenced on doxycycline, but he developed knee and elbow pains and stiffness after two weeks of treatment. He started with memory problems in 2006 and was seen by a psychiatrist in 2013. He completed 18 months of treatment with improvement in his anxiety, but his impaired memory persisted until the last follow-up in July 2024.
Case 16
A 38-year-old man presented with a nine-day history of fever (39.5 oC), severe headache and seven days of left side weakness, slurred speech, facial twitching, and dizziness. He also had multiple syncopal attacks during those nine days. A brain CT showed a 3 cm calcified lesion in the right occipital region, likely to represent a benign lesion and not explaining his symptoms. His serology was positive for phase II IgM. The patient received 18 months of HCQ and doxycycline with a resolution of his headaches and other neurological symptoms.
Case 17
A 57-year-old woman presented with a one-month history of intermittent fever (38.5 oC), palpitations, sweating, undocumented weight loss , and two days of dyspnea. Her medical history included Graves’ disease and atrial fibrillation, but she had defaulted three years ago, stopping all medications. Upon presentation, she had thyrotoxicosis and was positive for parainfluenza 1. Her fever and palpitations resolved upon the commencement of carbimazole. Her Q fever PCR and serology (sent initially for fever) were positive. Her ECHO showed mitral valve vegetation. She was commenced on doxycycline and HCQ, planned for a minimum of 18 months for infective endocarditis (IE). Unfortunately, she defaulted again five months after the presentation.
Discussion
Q fever was first reported in 1935 during an outbreak of febrile illness with flu-like symptoms in abattoir workers in Queensland, Australia. It was named ‘Q fever’ as a query because the etiology was not initially identified.3
The prevalence of Q fever in Oman remains unknown. In 2003, Scrimgeour et al,4 reported a 9.8% seroprevalence positivity in a human population (n = 102) presenting to Sultan Qaboos University Hospital for various diseases, such as diabetes mellitus and ischemic heart disease. Seropositivity in 52 randomly selected healthy goats reached up to 52%.4 In Saudi Arabia, human seroprevalence was similar to Oman at 8%, while animal seropositivity was lower at 30.7%.5,6 Q fever has a large reservoir in nature, including livestock, pets, birds, and marine mammals.7,8 Transmission routes include contact with infected animals, consumption of raw dairy products, tick bites, blood transfusions, vertical transmission from mother to fetus, and possibly sexual contact.1,9
Over a 15-year period, we identified 17 patients that were treated for Q fever based on their presentations and test results. The median age was 38 years (range = 13–59), and the male to female ratio was 1.4:1, similar to the previously reported ratio of 2.5:1.10 Patients were from various governorates in Oman including Muscat, Al Batinah, A'Sharqiyah, A'Dakhiliyah, A'Dhahirah and Dhofar (4, 4, 4, 2, 2, and 1 patients, respectively). Occupational history was obtained from 12 patients; however, it was missed for the remaining five. Among those, one patient was a camel jockey, a profession associated with Q fever seropositivity in camels, suggesting a possible source of infection. 11,12 The history of animal contact or raw dairy consumption was positive in six, negative in three, and missing in eight patients. Case two had a dog coughing, but its relevance to Q fever was not explored.
Q fever can manifest in acute or chronic forms. The acute form usually occurs 2–5 weeks post-exposure and ranges from asymptomatic infections (60%), and self-limited flu-like illness, to severe disease, such as pneumonia or hepatitis.13 Atypical acute presentations include perimyocarditis, neurological or hematological involvement, and aseptic meningitis.14 Chronic disease occurs in < 5% and develops months or years later.15 Manifestations include endovascular infections in the form of IE and infections of vascular prosthetic devices, granulomatous hepatitis, osteomyelitis, and interstitial pulmonary fibrosis.15–17 IE has also been reported in acute Q fever.18
Fever was the most common clinical feature in our cohort (88.2%), with some patients experiencing temperatures as high as 40°C, often accompanied by sweating and chills. Hepatitis, presenting as transaminitis, was seen in 10 (58.8%) patients, with four to five patients exhibiting high bilirubin levels (19, 56, 79, 240, 320 μmol/L) and clinically evident jaundice, totaling 12 (70.6%) patients, similar to the previously reported rate of 61.8%.19 Weight loss occurred in six (35.3%) patients. Splenomegaly, which is reported in 5% of acute Q fever cases, was observed in 11.8% of our cohort.20,21 Respiratory symptoms, including sore throat, dyspnea, cough, and chest pain, were reported by six (35.3%) patients, three of them had co-infections with influenza A, parainfluenza 3, and parainfluenza 1 (cases 4, 10, and 17, respectively). Ten patients had normal chest imaging, one had pulmonary edema, one had nodules, three had minimal pleural effusion, and one had consolidation summing up the pulmonary involvement to 35.3%, lower than previously reported of 45.8%.19 Some respiratory symptoms may be attributed to co-infections with other pathogens.
Neurological symptoms were reported in five (29.4%) patients, including severe headache, poor memory, focal neurological signs, involuntary movements, and seizures, which is lower than the 40.9% previously reported in acute Q fever.22 Case three presented six years after the initial presentation with a stroke at the age of 41 years, possibly indicating a relapse of Q fever, but this was not explored at the time.23 Case 16 had severe headache with a normal cerebrospinal fluid, excluding meningitis, and a brain CT scan showed a 3 cm calcified lesion unlikely to cause symptoms. He was asymptomatic after completing 18 months of treatment. A repeat CT scan was planned in due course.
Four (23.5%) patients presented with musculoskeletal symptoms in the form of sacroiliitis and asymmetric small joints of hands arthralgia, much higher than the previously reported 2%.24 Three patients developed new pains in their knees, ankles, and Achilles tendon during or after completion of treatment, possibly due to the release of lipopolysaccharides from bacterial killing, similar to gram-negative bacterial infections. Lipopolysaccharides is a known virulence factor for Coxiella, varying in different phases of the virulent phase I.25 Three (17.6%) patients developed maculopapular or purpuric rashes consistent with the reported 5–21% incidence of skin rashes.26 It is unclear whether these rashes were part of the Q fever or drug reactions. Two patients had hematological involvement, including bicytopenia and neutropenia, and one patient had lymphadenopathy.
Two patients had IE. Case 13, a patient with SLE, had a mass attached to the mitral valve with negative blood cultures. It was unclear whether this was Libman-Sacks endocarditis or culture-negative IE. The patient had no clinical or serological evidence of active SLE. The Libman-Sacks vegetations, typically small or medium-sized and located on either or both sides of the valve leaflet, differ from the large, irregular masses seen in IE, which often extend to the chordae tendinae.27 The patient’s Q fever serology was positive with high titers, and she was treated with doxycycline and HCQ for 2.5 years. A follow-up ECHO showed complete resolution of the vegetation with treatment. Case 17 had a history of Graves’ disease and atrial fibrillation and had defaulted from Q fever IE treatment after five months. Unfortunately, she passed away before completing her treatment. Case one was treated as probable IE, although the ECHO showed increased valvular leaflet thickening rather than vegetation. A transesophageal ECHO was not performed, and the patient was referred back to his local hospital without further follow-up. Case four had pericardial effusion that was eventually believed to be due to Q fever and not SLE flare as the complements were normal and the patient did not respond to steroids. She responded to doxycycline only as she was intolerant to HCQ.
White blood cell counts were elevated in four patients, all had coinfections (Achomobacter, influenza A, parainfluenza 3, and parainfluenza 1; cases 2, 4, 10, and 17, respectively). In the remaining cases, white blood cell was normal, consistent with other reports.28 C-reactive protein was significantly raised in 13 patients ranging from 50–336. Erythrocyte sedimentation rate was raised, but we could not continue monitoring its levels due to institutional policy changes.
Q fever diagnosis is primarily based on serology, as culturing the organism is hazardous and requires biosafety level 3 protection, which is not available in all laboratories. Indirect fluorescent antibodies testing is the gold standard for providing antibody titers. During natural human infection, antibodies are produced in a time sequence to phase II and I antigens. In acute infection, antibodies react to phase II antigens, while in chronic infection, they react to a mixture of phase I and II antigens. IgM and IgG anti-phase II antibodies are detectable 2–3 weeks after infection. Detection of IgG I at titers ≥ 1:800 by microimmunofluorescence assay indicates chronic Q fever.20 At our institution, Q fever testing is done using a commercial qualitative enzyme-linked immunosorbent assay test (Institut Virion/Serion Gmbh, Germany). While useful as an initial screening test, it cannot solely diagnose Q fever, particularly in chronic infection. The presence of RFs in patient samples can cross-react with Q fever IgM assays, leading to false positives. In our cohort, case two and three had positive RF and ACPA at presentation, which turned negative after Q fever treatment, indicating potential false positives. Cross-reactivity of Q fever serology with other infections, such as Legionella and Bartonella, has been described depending on the test used, adding to diagnostic confusion, but this can be resolved with titer determination.20 NAATs, such as PCR, can diagnose acute Q fever within the first two weeks of infection, where it is more sensitive than serology but rapidly declines thereafter. However, NAATs can also diagnose chronic Q fever using tissue samples, such as heart valves in IE cases.29
The treatment of Q fever depends on its presentation. The treatment of the complex chronic form includes doxycycline (100 mg twice daily) and HCQ (200 mg three times daily) for 12–18 months.30 However, in cases four and six, doxycycline was given alone due to HCQ intolerance. Although alternative agents, such as quinolones, can be used, we were unaware of the HCQ noncompliance until near the end of the treatment, so the patients continued on doxycycline alone and responded, an observation that merits further investigation.
In the recent study, Q fever testing was not based on a history of animal contact or raw dairy consumption, as Q fever has been reported in cases without such histories.31,32 Transmission can occur through inhalation of spores from areas where previously infected animals have shed their placenta, with spores capable of traveling over 30 km.33 Spores are resistant to common environmental conditions, such as heat, drying, and some common disinfectants, and can survive up to 120 days. In addition, Q fever is the most infectious bacteria with only one bacterium being sufficient to cause the illness.34 We tested a total of 1481 patients, but only 17 were treated for Q fever. This significant difference highlights the need to consider Q fever in differential diagnoses while also showing the high cost of diagnosing one case. Additionally, qualitative positive serology alone is insufficient to establish a diagnosis, especially in endemic areas.
We noted that Q fever was a co-infection with another illness in more than one case, such as in case 17, where the initial symptoms could have been attributed to thyrotoxicosis. Whether this is a coincidence or reflects an underestimated Q fever prevalence needs further investigation. Despite multiple qualitative serology tests, doubts remained in establishing diagnoses in some cases, which were later given alternative diagnoses, such as Behçet’s disease, seronegative rheumatoid arthritis, and autoimmune hepatitis. Additionally, the diagnosis of case five remains unclear, as investigations were not suggestive of hemophagocytic lymphohistiocytosis, blood cultures were negative, and tests for antinuclear antibodies and lupus anticoagulant were negative. Unfortunately, post-mortem examination is not practiced in our region due to religious beliefs. Recently, we began sending some samples for indirect fluorescent antibodies testing abroad after initial positive qualitative enzyme-linked immunosorbent assay results, depending on physician requests. PCR is now available at a local reference laboratory, improving our diagnostic capabilities. Our study illustrates that Q fever should be considered in a wide range of clinical presentations, but over-testing can lead to unnecessary costs. Adopting diagnostic algorithms for Q fever, both clinical- and laboratory-based, may further improve diagnostic accuracy.
The unavailability of the appropriate laboratory tests when needed was a major limitation in this cohort. Other limitations included the lack of patients’ continuity of care due to their transfer to other hospitals or default.
Conclusion
Q fever should be considered as a potential differential diagnosis in many clinical presentations. Appropriate laboratory tests for diagnosis should be discussed with the medical microbiologists, and results should be interpreted in the context of the patient’s clinical presentation.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
references
- 1. Centers for disease control and prevention. About Q fever. 2024 [cited 2023 December 20]. Available from: cdc.gov/qfever/index.html.
- 2. Körner S, Makert GR, Ulbert S, Pfeffer M, Mertens-Scholz K. The prevalence of Coxiella burnetii in hard ticks in Europe and their role in Q fever transmission revisited-a systematic review. Front Vet Sci 2021 Apr;8:655715.
- 3. Derrick EH. “Q” fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Med J Aust 1937;2:281-299.
- 4. Scrimgeour EM, Al-Ismaily SI, Rolain JM, Al-Dhahry SH, El-Khatim HS, Raoult D. Q fever in human and livestock populations in Oman. Ann N Y Acad Sci 2003 Jun;990:221-225.
- 5. Alhetheel AF, Binkhamis K, Somily A, Barry M, Shakoor Z. Screening for Q fever. A tertiary care hospital-based experience in central Saudi Arabia. Saudi Med J 2018 Dec;39(12):1195-1199.
- 6. Jarelnabi AA, Alshaikh MA, Bakhiet AO, Omer SA, Aljumaah RS, Harkiss GD, et al. Seroprevalence of Q fever in farm animals in Saudi Arabia. Biomedical Research-tokyo 2018;29(5):895-900.
- 7. Marrie TJ, Durant H, Williams JC, Mintz E, Waag DM. Exposure to parturient cats: a risk factor for acquisition of Q fever in Maritime Canada. J Infect Dis 1988 Jul;158(1):101-108.
- 8. Kersh GJ, Fitzpatrick K, Pletnikoff K, Brubaker M, Bruce M, Parkinson A. Prevalence of serum antibodies to Coxiella burnetii in Alaska Native Persons from the Pribilof Islands. Zoonoses Public Health 2020 Feb;67(1):89-92.
- 9. Buhariwalla F, Cann B, Marrie TJ. A dog-related outbreak of Q fever. Clin Infect Dis 1996 Oct;23(4):753-755.
- 10. Angelakis E, Raoult D. Emergence of Q fever. Iran J Public Health 2011;40(3):1-18.
- 11. Holloway P, Gibson M, Nash S, Holloway T, Cardwell J, Al Omari B, et al. A cross-sectional study of Q fever in camels: risk factors for infection, the role of small ruminants and public health implications for desert-dwelling pastoral communities. Zoonoses Public Health 2023 May;70(3):238-247.
- 12. Abdelfattah S, Ali A-F. Seroprevalence and risk factors for C. burentii infection in camels in Egypt. Comp Immunol Microbiol Infect Dis 2020 Feb:68:101402.
- 13. Alves J, Almeida F, Duro R, Ferraz R, Silva S, Sobrinho-Simões J, et al. Presentation and diagnosis of acute Q fever in Portugal - A case series. IDCases 2016 Dec;7:34-37.
- 14. Gu M, Mo X, Tang Z, Tang J, Wang W. Case report: diagnosis of acute Q fever with aseptic meningitis in a patient by using metagenomic next-generation sequencing. Front Med (Lausanne) 2022 May;9:855020.
- 15. Das I, Guest N, Steeds R, Hewins P. Chronic Q fever: an ongoing challenge in diagnosis and management. Can J Infect Dis Med Microbiol 2014;25(1):35-37.
- 16. van Roeden SE, Wever PC, Kampschreur LM, Gruteke P, van der Hoek W, Hoepelman AI, et al. Chronic Q fever-related complications and mortality: data from a nationwide cohort. Clin Microbiol Infect 2019 Nov;25(11):1390-1398.
- 17. Patil SM, Regunath HQ. Fever. [Updated 2022 Nov 18]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
- 18. Melenotte C, Epelboin L, Million M, Hubert S, Monsec T, Djossou F, et al. Acute Q fever endocarditis: a paradigm shift following the systematic use of transthoracic echocardiography during acute Q fever. Clin Infect Dis 2019 Nov;69(11):1987-1995.
- 19. Tissot Dupont H, Raoult D, Brouqui P, Janbon F, Peyramond D, Weiller PJ, et al. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. Am J Med 1992 Oct;93(4):427-434.
- 20. Maurin M, Raoult D. Q fever. Clin Microbiol Rev 1999 Oct;12(4):518-553.
- 21. Raoult D, Houpikian P, Tissot Dupont H, Riss JM, Arditi-Djiane J, Brouqui P. Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med 1999 Jan;159(2):167-173.
- 22. Kofteridis DP, Mazokopakis EE, Tselentis Y, Gikas A. Neurological complications of acute Q fever infection. Eur J Epidemiol 2004;19(11):1051-1054.
- 23. Vinacci G, Tcheumeni CN, Coskun O, Saddiki FZ, Maria FD, Dinu M, et al. White thrombus as a possible feature of atypical stroke etiology: Coxiella burnetii as the primary cause of acute ischemic stroke. Interv Neuroradiol 2022 Apr;28(2):142-144.
- 24. Alder KD, Fiegen AP, Rode MM, Tai DB, Suh GA, Virk A, et al. Chronic Q fever presenting as bilateral extensor tenosynovitis: a case report and review of the literature. J Bone Jt Infect 2023 Jan;8(1):39-44.
- 25. Abnave P, Muracciole X, Ghigo E. Coxiella burnetii lipopolysaccharide: what do we know? Int J Mol Sci 2017 Nov;18(12):2509.
- 26. Ergas D, Keysari A, Edelstein V, Sthoeger ZM. Acute Q fever in Israel: clinical and laboratory study of 100 hospitalized patients. Isr Med Assoc J 2006 May;8(5):337-341.
- 27. Moyssakis I, Tektonidou MG, Vasilliou VA, Samarkos M, Votteas V, Moutsopoulos HM. Libman-Sacks endocarditis in systemic lupus erythematosus: prevalence, associations, and evolution. Am J Med 2007 Jul;120(7):636-642.
- 28. de Wit NC, de Jager CP, Meekelenkamp JC, Schoorl M, van Gageldonk-Lafeber AB, Leenders AC, et al. Markers of infection in inpatients and outpatients with acute Q-fever. Clin Chem Lab Med 2009;47(11):1407-1409.
- 29. Jang YR, Song JS, Jin CE, Ryu BH, Park SY, Lee SO, et al. Molecular detection of Coxiella burnetii in heart valve tissue from patients with culture-negative infective endocarditis. Medicine (Baltimore) 2018 Aug;97(34):e11881.
- 30. van Roeden SE, Bleeker-Rovers CP, de Regt MJ, Kampschreur LM, Hoepelman AI, Wever PC, et al. Treatment of chronic Q fever: clinical efficacy and toxicity of antibiotic regimens. Clin Infect Dis 2018 Feb;66(5):719-726.
- 31. Vellema P, van den Brom R. The rise and control of the 2007–2012 human Q fever outbreaks in the Netherlands. Small Rumin Res 2014;118:69-78.
- 32. Mori M, Mertens K, Cutler SJ, Santos AS. Critical aspects for detection of Coxiella burnetii. Vector Borne Zoonotic Dis 2017 Jan;17(1):33-41.
- 33. Tissot-Dupont H, Amadei MA, Nezri M, Raoult D. Wind in November, Q fever in December. Emerg Infect Dis 2004 Jul;10(7):1264-1269.
- 34. Marrie TJ. Q fever - a review. Can Vet J 1990 Aug;31(8):555-563.