Respiratory syncytial virus (RSV) accounts for significant morbidity and mortality burden on a global scale among children younger than five years of age, with the greatest burden in infants aged under six months. A systematic literature review (2017–2020) documented nearly 33 million episodes of RSV-associated acute lower respiratory tract infections (LRTIs) globally in children aged ≤ 5 years, resulting in 3.6 million hospitalization events and 26 300 in-hospital deaths.1 Approximately half of these RSV-related hospital admissions (1.4 million) and inhospital deaths (13 300) were reported among infants aged ≤ 6 months. More than 97% of the RSV-attributable mortality occurred in low- and middle-income countries.1 The RSV-related LRTIs during early childhood can lead to long-term respiratory sequelae such as recurrent wheezing, asthma, and impaired lung function.2 Premature infants and children with pre-existing cardiac, pulmonary, neuromuscular, and immunosuppressive disorders have greater susceptibility to developing severe RSV.3 Several environmental and host-related risk factors like male gender, low birth weight, poor socioeconomic status, younger siblings, daycare attendance, lack of breastfeeding, and family history of atopy can predispose healthy children to severe RSV infection.4,5 The RSV infection displays a seasonal transmission pattern with distinct regional and geographical variability, with marked winter-spring predominance (between October and May) in temperate countries and greater interseasonal variability with lesser pronounced spikes in the tropics.6 In the Gulf Cooperation Council (GCC) region, RSV is highly prevalent between August and February, peaking in winter months (December and January) and decreasing during March and July.7
Epidemiological evidence suggests a high prevalence of RSV infection in the GCC region with a wide variability in the rates of RSV incidence.7–12 In the light of considerable burden, preventing RSV LRTIs in infants is a major public health priority. Currently, palivizumab, a monoclonal antibody (mAb), is a widely used passive immunization preventive strategy against RSV for high-risk infants and young children. It targets F protein, crucial for virus attachment and fusion, thereby neutralizing the virus and preventing its entry into the cells.13 However, its widespread use is limited due to substantial expense and monthly dosing requirements despite a well-proven efficacy in reducing RSV-related hospitalizations. Recently, newer alternatives like nirsevimab (longer-acting, single-dose mAb) are recommended for passive immunization against RSV.14 A significant shift in RSV management is expected in the coming years, but prioritizing reinforcement of palivizumab prophylaxis is crucial based on the accessibility of novel treatments within the region.
In the GCC region, several national-level recommendations for RSV immunoprophylaxis have been developed in alignment with local epidemiological data.15,16 Yet, due to the lack of regional directives, some countries still adhere to the American Academy of Pediatrics (AAP) guidelines.17 However, effective RSV management requires a combination of evidence-based recommendations, regional surveillance, and medical practices, suggesting an urgent need to align guidelines for RSV immunoprophylaxis across the GCC region. This paper intends to provide an overview of the regional and country-specific burden of RSV, identify challenges in the utilization of palivizumab, and provide expert recommendations in facilitating the optimization of RSV immunoprophylaxis programs across the region.
Methods
The concept of formulating a GCC steering committee meeting was convened in June 2023 to discuss the epidemiology and burden of RSV, current unmet needs, and challenges in effective RSV management in the GCC region. It was decided to work with experts from across the GCC to share their collective opinions on current RSV immunoprophylaxis practices, obstacles to compliance, and measures to enhance compliance with palivizumab prophylaxis. Evidence on the prevalence of RSV, the efficacy of palivizumab immunoprophylaxis, and optimal timing for initiating RSV immunoprophylaxis programs were compiled. Based on their clinical experience and the latest published evidence on the efficacy of palivizumab prophylaxis in high-risk infants, the experts shared practical recommendations to address existing gaps and to facilitate local physicians in effective RSV management in the region. These recommendations may serve as a unified reference guide for healthcare practitioners (HCPs), RSV program directors, and those involved in recruiting eligible patients in RSV immunoprophylaxis programs, thus allowing optimal utilization of resources and cost-effective practices across the region.
Prevalence and disease burden in the GCC region
Despite the considerable burden of RSV, there is a paucity of epidemiological studies evaluating its prevalence in the GCC countries. In Saudi Arabia, the prevalence of RSV among young children < 5 years of age experiencing acute LRTIs ranged from 0.2% to 70.2% from 1991 to 2018.9 A more recent study from Saudi Arabia (2015–2022) revealed a high RSV infection rate of 56.8% in children ≤ 5 years.7 A systematic review of RSV-related evidence from 2001 to 2019 found Qatar had a higher annual RSV incidence rate of 48.5% (2010–2011), whereas Oman had the lowest incidence at 1.8% (2011–2012).8 In Bahrain (2018–2021), RSV was the third most prevalent viral infection (14.3%), after Flu-A (37.5%) and SARS-CoV-2 (33%).12
Regional data indicated a higher prevalence of RSV infections among children < 12 months of age, particularly among males.8,18 Preterm infants with comorbid conditions like chronic lung disease (CLD), bronchopulmonary dysplasia (BPD), and hemodynamically significant-congenital heart disease (CHD) exhibited increased susceptibility to RSV infection. Other risk factors included multiple births, siblings attending school or daycare, exposure to tobacco smoking, daycare environments, and a family history of asthma.19,20 RSV infections tend to be more common during the winter season, indicating a strong seasonal activity of the virus.7 According to the phylogenetic analysis studies, the prevalent Saudi strains of group-A RSV can be classified into the NA1 and ON1 genotypes, while the group B-RSV tends to cluster within the BA genotype.9,21,22 The RSV-A subgroup was more dominant than the RSV-B subgroup.9,22 A study conducted in Kuwait investigating genetic variations in the RSV strains prevailing during the 2016 season found a predominance of RSV-A (67.5%) over the RSV-B subgroup (32.5%). While the circulating strains of the RSV-A group were new and untyped, that did not align with any of the known group-A genotypes, most of the RSV-B group strains belonged to the BA10 genotype.23
Evidence on the efficacy of palivizumab immunoprophylaxis in a high-risk population
early preterm infants
Preterm infants exhibit a higher incidence of RSV infection and subsequent hospitalization.24 Among preterm infants, those born at < 29 weeks gestational age (wGA) are more susceptible to experiencing severe RSV infection, which can result in extended duration of hospitalization and increased healthcare costs.25 The current AAP recommendations advocate palivizumab prophylaxis for infants born at < 29 wGA who are < 12 months of age at the onset of RSV season [Box 1]. Additionally, infants born at ≥ 29 wGA may qualify for RSV prophylaxis based on the presence of certain high-risk conditions, such as CLD or BPD.17
Box 1: Expert recommendations for palivizumab immunoprophylaxis in early preterm infants.
|
Consistent with the current international guidelines, the experts recommend palivizumab prophylaxis to infants born before 29 wGA and under 12 months of age at the start of the RSV season.
|
preterm children with cld and bpd
Palivizumab prophylaxis has been proven effective in children with CLD or BPD. According to a meta-analysis, palivizumab prophylaxis resulted in a 65% reduction in RSV hospitalization, compared to untreated infants.26 A Cochrane database review concluded that palivizumab prophylaxis effectively reduced RSV-related hospitalizations among
patients with CLD.27 Additionally, the Canadian CARESS study revealed that children receiving palivizumab had similar rates of RSV hospitalization in the first and second years of life (hazard ratio (HR) = 1.1; 95% CI: 0.4–2.9; p = 0.920).28 Paes and Estrany suggested considering palivizumab prophylaxis in the first two years for all children with CLD, regardless of the severity of the disease.29 Box 2 provides the expert recommendations for managing RSV in infants with CLD or BPD.
Box 2: Expert recommendations for palivizumab immunoprophylaxis in children with chronic lung disease (CLD) and bronchopulmonary dysplasia (BPD).
|
Palivizumab is recommended for all patients with CLD or BPD who are below 12 months of age and can be extended during the second season for those who continue to receive CLD medications within 6 months at the onset of the RSV season.
|
children with congenital heart abnormalities
Palivizumab prophylaxis for children with hemodynamically significant CHD resulted in a reduction of 45% in hospital admissions due to RSV (p = 0.003), 56% in total days of RSV-related hospitalizations (p = 0.003), and 73% in the total number of RSV-related hospital days requiring supplemental oxygen (p = 0.014).30 Another study demonstrated a 19% reduction in the frequency of RSV hospitalizations following palivizumab prophylaxis among children with CHD compared to the pre-prophylaxis period (2000 to 2002).31 Chiu et al,32 documented a significant decline in the RSV hospitalization rate in patients with hemodynamically significant CHD following palivizumab prophylaxis by 53% and 49%, before and after match comparison with the control group (p = 0.009 and p = 0.029, respectively). Additionally, palivizumab recipients had a shorter duration of hospitalization and a lower rate of intensive care unit (ICU) admission. Efficacy outcomes were more pronounced in patients with cyanotic hemodynamically significant CHD. Moreover, there was a reduction in the annual rate of RSV-related hospitalization from pre-palivizumab to post-palivizumab period (4.8% vs. 2.0%; p = 0.038).32
In line with global findings, a study conducted in Saudi Arabia assessed the efficacy of palivizumab prophylaxis among 530 children with hemodynamically significant CHD, cyanotic CHD, and moderate-to-severe pulmonary hypertension. Throughout six RSV seasons (2010–2016), only 13 (2.5%) patients required RSV-related hospitalization, with only one patient necessitating ICU admission. Importantly, no adverse events or deaths were attributed to RSV during the study period.33
In the CARESS registry data (2005–2015), the risk of RSV-related hospitalization (HR = 2.0; 95% CI: 0.2–16.5; p = 0.52) and respiratory illness-related hospitalization (HR = 1.9; 95% CI: 0.7–4.6; p = 0.18) were found to be similar for the first and second year of life. Also, the second-year infants revealed a more complicated disease course with a significantly longer duration of hospitalization (51.2 vs. 24.9 days in the first season).34 Box 3 provides the expert's recommendation for infants with hemodynamically significant CHD and children with hemodynamically unstable cardiac conditions.
Box 3: Expert recommendations for palivizumab immunoprophylaxis in children with congenital heart abnormalities.
|
Palivizumab prophylaxis is recommended for all infants under 12 months with hemodynamically significant CHD (cyanotic or acyanotic). It is also recommended for children aged 12 to 24 months who remain hemodynamically unstable and are still on medication for cardiac conditions six months prior to the start of the epidemic season.
Infants who undergo cardiopulmonary bypass in the current RSV season are recommended to receive an additional dose of palivizumab. The dose should be administered promptly once the infant is stable after the procedure, even if it is within a month of the previous dose; subsequent doses should be given on a monthly basis as scheduled. This recommendation is based on a 58% reduction in the serum concentration of palivizumab after such procedures. Children younger than two years undergoing cardiac transplantation during the RSV season may be considered for palivizumab prophylaxis.
|
children with neuromuscular disorders, anatomic abnormalities, and immunodeficiency
Currently, there is limited evidence supporting the efficacy of palivizumab prophylaxis among subpopulations such as children with pulmonary malformations, anatomical lung abnormalities with impaired lower airway clearance, severe upper airway obstruction, immunodeficiency, metabolic disorders, congenital diaphragmatic hernia, and lung transplantation. Table 1 summarizes the evidence regarding the effectiveness of palivizumab in children with Down's syndrome, cystic fibrosis, and those with severe immunodeficiency.35–40 Despite the lack of conclusive evidence in these patients, in the experts’ view, these patient categories are likely to benefit from RSV immunoprophylaxis [Box 4].
Table 1: Effectiveness of palivizumab on RSV-related hospitalization in children with Down’s syndrome (DS), cystic fibrosis, and severe immunodeficiency.
|
Studies on effectiveness of palivizumab in children with DS
|
|
Paes et al,35 2014
|
CARESS prospective registry (2006–2012)
|
High-risk infants receiving at least 1 dose of palivizumab
|
13 310 (of which 600 children had DS)
|
RSV hospitalization rate for children with DS who received prophylaxis (1.53%) was similar to children with other standard indications (1.45%).
|
|
Kimura et al, 36 2020
|
2007–2015
|
≤ 2 years
|
632
Palivizumab = 384
Control = 248
|
RSV-related hospitalization occurred in 4.2% patients with prophylaxis and 6.0% patients without prophylaxis.
Palivizumab led to significant reduction in RSV-related hospitalization (odds ratio = 0.41, 95% CI: 0.18–0.92; p = 0.03).
|
|
Studies on effectiveness of palivizumab in children with cystic fibrosis
|
|
Kua and Lee, 37 2017
|
Systematic review
|
< 2 years
|
3891
|
Palivizumab prophylaxis reduced the risk of RSV hospitalization.
|
|
Sãnchez-Solis et al,38 2015
|
Random-effects meta-analysis
|
|
Palivizumab = 354
Untreated = 463
|
Palivizumab prophylaxis significantly reduced the hospitalization rate, compared to untreated group (0.018 vs. 0.126, respectively; p < 0.001).
|
|
Fink et al,39 2019
|
Cystic Fibrosis Foundation Patient Registry data
(2008–2015)
|
≤ 2 years
|
4267 (of which 1588 received palivizumab)
|
Patients receiving prophylaxis showed similar long-term outcomes (pulmonary function, annual risk of hospitalization, or time to first positive sputum culture), compared to those who did not receive palivizumab.
|
|
Effectiveness of palivizumab in immunocompromised children
|
RSV: respiratory syncytial virus.
Box 4: Expert recommendations for palivizumab immunoprophylaxis in high-risk patient populations.
|
Although conclusive evidence is lacking for these high-risk populations, the expert panel recommends palivizumab immunoprophylaxis for the following conditions:
- Down’s syndrome: recommended for children with concomitant qualifying heart disease, CLD, airway obstruction, with inability to clear airway due to weak cough, or those born prematurely (< 35 wGA).
- Cystic fibrosis: < 12 months for infants with CLD and/or nutritional deficiency; < 24 months for those with preclinical or clinical evidence of severe CLD (based on computed tomography or radiological findings on admission) OR weight for length below 10th percentile.
- Children with anatomic pulmonary abnormalities or neuromuscular disorders: < 24 months with difficulty in managing respiratory secretions.
- Children with severe immunodeficiency: < 24 months during the RSV season.
|
rsv prophylaxis in moderate- and late-preterm infants
From 2009 to 2012, the AAP advocated palivizumab prophylaxis to all preterm infants born at < 32 wGA, and those born from 32 to < 35 wGA, and < 3 months of chronological age at the onset of RSV season with at least one additional risk factor such as childcare attendance or living with a sibling under five years of age in the same household.41 However, in 2014, the AAP discontinued recommending palivizumab for infants born ≥ 29 wGA unless they had specific comorbidities.17 Subsequent studies evaluating the impact of revised AAP recommendations demonstrated a notable reduction in palivizumab use and a concurrent increase in the risk of RSV hospitalization along with higher disease severity and utilization of healthcare resources among infants born between 29–35 wGA.42,43
predictive model for risk factors in infants born 29–35 wga
Researchers have highlighted the vulnerability of young preterm infants, and a need to reevaluate palivizumab prophylaxis in the > 29 wGA subpopulation based on specific risk factors.44 Several guidelines such as those from Spain,45 Italy,46 the Netherlands,47 and Canada48 have adopted risk-scoring tools (RST) to assess the risk of RSV hospitalization based on predetermined risk factors, thus allowing for targeted and cost-effective prophylaxis. Blanken et al,48 developed an international (IRST) to predict the risk of RSV hospitalization in moderate and late preterm infants (32–35 wGA) based on the risk factors such as proximity of birth to RSV season (birth between three months before and two months after the start of RSV season), second-hand smoke exposure in the household or smoking during pregnancy, and siblings and/or day care.49 The IRST showed high accuracy at predicting RSV-related hospitalization (area under the receiver operating characteristic curve (AUROC) = 0.773, sensitivity = 68.9%, specificity = 73.0%).49
Implementation of IRST with fewer risk factors has displayed a comparable predictive accuracy to the Canadian 7-variable RST (AUROC (IRST = 0.773, sensitivity = 68.9%, specificity = 73.0%) vs. Canadian RST (CRST) = 0.762, (68.2%, 71.9%)) among moderate to late preterm infants (32–35 wGA). While the percentage of high-risk infants was similar for IRST (0.7%) and CRST (0.6%); the latter demonstrated a lower number needed to treat (7.5 vs. 14.3), and fewer infants classified as moderate risk (9.8% vs. 19.9% for CRST and IRST, respectively).50 Additionally, the cumulative risk scores obtained from the CRST and the IRST are moderately correlated (rs = 0.64; p < 0.001).51 Moreover, a cost-utility analysis demonstrated palivizumab to be highly cost-effective when administered to Canadian moderate to late preterm infants identified with moderate and high risk of RSV hospitalization using IRST, compared to without prophylaxis.52
long-term respiratory outcomes after rsv infection in infants born 29–35 wga
There is compelling literature evidence indicating that severe RSV infection during infancy in premature infants may cause long-term respiratory sequelae in later childhood. A Scottish study revealed that previous RSV-related hospitalization between six to 23 months of age was strongly associated with subsequent development of wheezing and asthma at two years of follow-up, which gradually decreased over time and persisted until age six.53 A systematic review and meta-analysis established a significant association between early-life RSV infection and recurrent wheeze and asthma in children aged six to 12 years at follow-up.54 Additionally, caregivers for infants born at 29–35 wGA and who were hospitalized due to confirmed RSV infection, have been reported to experience significant stress during hospitalization, which continued until one-month post-discharge. Allied with this, RSV hospitalization is also associated with several socioeconomic implications, such as missed work or productivity loss, financial burden, disruption of family health and routine, separation from siblings, and strained family relationships.55
Studies have suggested that palivizumab prophylaxis for RSV infection reduced subsequent wheezing in premature infants (≤ 35 wGA).26,56–58 The MAKI trial showed a nearly 50% reduction in recurrent wheezing among palivizumab recipients than placebo (11% vs. 21%; p = 0.01) during the first year of life.56 Subsequent follow-up of this study, at the age of six revealed a reduction in parent-reported current asthma among infants treated with palivizumab than the control group (absolute risk reduction = 9.9%; 95% CI: 2.2–17.6).57 These findings were supported by the Japanese CREW study, which revealed a lower rate of physician-diagnosed recurrent wheezing in palivizumab recipients relative to untreated patients, at the age of three years (6.4% vs. 18.9%; p < 0.001).58 Box 5 provides the expert recommendations for managing RSV in moderate and late preterm infants.
Box 5: Expert recommendations for palivizumab immunoprophylaxis in moderate to late preterm infants.
|
For moderate preterm infants (born 290–326 wGA) with chronological age of ≤ 6 months at the start of the RSV epidemic. For late preterm infants (330 to 350) wGA: ≤ 6 months when the RSV season begins or if they are born during the season and have any of the specified risk factors such as attending childcare, residing permanently with siblings or children under five years old in the same household, and contact with environmental air pollutants.
|
Impact of covid on RSV seasonality, disease course, and outcomes
Regional differences in the duration and timing of the RSV season are influenced by demographics, climatic conditions, and population density. Stringent public health measures implemented during the COVID-19 pandemic led to a dramatic reduction in RSV incidence during the usual epidemic season. However, with relaxation of restrictions, definitive shifts in the RSV seasonality pattern were observed, delaying the onset of the RSV season.59 Subsequently, the reemergent RSV outbreaks were more severe and affected a broader patient population than in typical RSV seasons.
In Qatar, RSV incidence decreased from 21.2% in 2019 to 0.7% in 2020 but returned to typical pre-pandemic levels (22.3%) in 2021 following relaxation of pandemic restrictions.60 In Saudi Arabia, RSV infections were more common from August to February, peaking from December to January. No RSV cases were reported during the COVID-19 pandemic. However, in August 2021, the number of RSV-positive cases experienced a two-fold rise, compared to previous years.7
In response to shifting RSV epidemiology during the COVID-19 pandemic, specialized task forces reconsidered the criteria for palivizumab prophylaxis. Since 2021, the AAP recommended palivizumab prophylaxis in eligible patients during interseasonal RSV spread and considered providing more than five consecutive doses of palivizumab depending on the RSV seasonality duration in a region.61 In the UK, the RSV immunoprophylaxis programs have also been modified, allowing eligible children to start early in July (instead of October) and implementing a seven-monthly dosing regimen.62
The Saudi Pediatric Pulmonology Association faced several challenges in conducting RSV immunoprophylaxis programs amid COVID-19 period. Due to increased strain on healthcare facilities, infants particularly those with compromised immune system became more susceptible to RSV. Additionally, concerns about contracting COVID-19 in hospitals contributed to non-adherence to medical recommendations. Re-engagement of high-risk infants born before the pandemic further complicated the immunoprophylaxis initiatives.63 To its response, several changes in clinical practices were implemented, including increasing RSV clinics, extending operational days, setting up drive-through facilities, scheduling appointments, implementing home vaccinations to limit COVID-19 exposure, and expediting referrals to specialists.63 A specialized referral form was disseminated among HCPs, including pediatricians and staff in neonatal ICU and pediatric emergency rooms, to enhance awareness [Figure 1].15
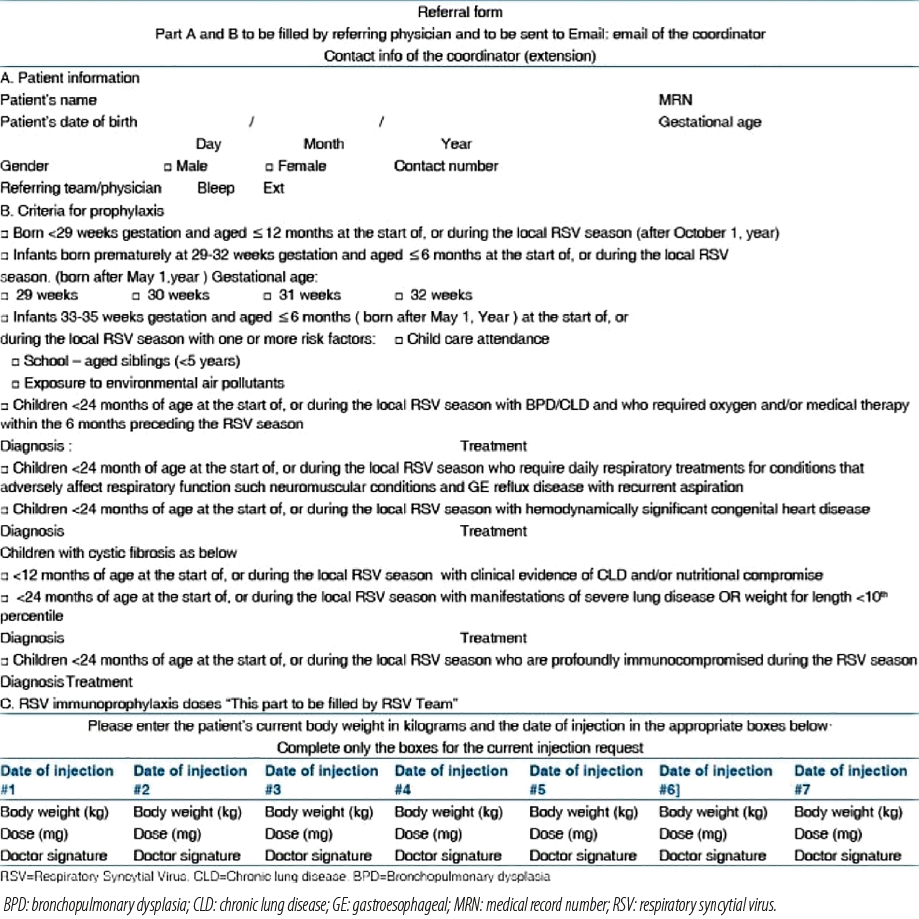 Figure 1: Referral form to facilitate patient enrollment in the RSV immunoprophylaxis program during COVID-19 pandemic. Permission conveyed through Copyrights Clearance Center.15
Figure 1: Referral form to facilitate patient enrollment in the RSV immunoprophylaxis program during COVID-19 pandemic. Permission conveyed through Copyrights Clearance Center.15
Overall, in the view of disrupted RSV activity in post-pandemic seasons, the experts recommend a flexible approach along with regular surveillance and frequent reassessment of immunoprophylaxis guidelines to mitigate future RSV surges.
Optimal timing for initiating RSV immunoprophylaxis programs
Anticipating the onset of RSV epidemic season is currently a challenge due to atypical seasonal pattern observed in the post-pandemic years. The experts recommended relying on local RSV surveillance data to better understand the trend of annual RSV incidence over the recent years and optimize the start month, duration of administration, and number of doses.
Optimizing the dosage and timing of palivizumab administration based on the local RSV season may lead to improved outcomes. A retrospective study conducted among the high-risk Saudi population (2009–2017) evaluated the effectiveness of three palivizumab regimens: a four-week interval dosing regimen starting in November (season 1), a four-week interval dosing regimen commencing in mid-September (season 2), and a three-week interval dosing regimen starting in mid-September for the remaining study duration. Although a decline in the RSV incidence rate was noted with the three-week interval regimen (3.9% vs. 5.9% and 9.1% in seasons 1 and 2, respectively), the differences among the three groups were statistically insignificant.18 A study from Qatar found that RSV-related hospitalizations peaked during November and December which coincided with the first and second dose interval, potentially attributed to the lower serum levels of palivizumab early in the prophylaxis regimen,20 suggesting to consider early initiation of the dosing schedule to align with the peak of RSV season. Consequently, the Saudi guidelines recommend starting the RSV immunoprophylaxis program early (preferably in mid-September). Regional experts also suggested maintaining a shorter interval between the two consecutive initial doses than the recommended interval. A Phase II study from Saudi Arabia during the 2001–2002 season exhibited favorable safety profile of seven monthly dosing regimen of palivizumab prophylaxis among high-risk children.64
Summary of recommendations for RSV immunoprophylaxis with palivizumab across different patient categories
Table 2 presents a comprehensive summary of recommendations for RSV immunoprophylaxis with palivizumab, across various patient populations.65–67 These recommendations serve as a practical guide for HCPs in determining the optimal use of palivizumab for RSV prevention in vulnerable populations in the GCC region.
Table 2: Expert recommendations for RSV immunoprophylaxis with palivizumab among different patient populations.
- Dosing: 15 mg/kg once a month during RSV season (minimum five doses), packaged in 100 mg vials, and the opened vials are recommended to be used within six hours.
- Administration: intramuscular injection, ideally in the anterolateral region of the thigh. The gluteal muscle is not recommended as a routine injection site due to the potential risk of sciatic nerve damage. Administration should adhere to standard aseptic procedures.65
- Early initiation of the RSV immunoprophylaxis program is recommended. Regional experts suggested maintaining a shorter duration between the initial two doses followed by regular interval of four weeks in subsequent doses.
- Depending upon the severity or interseasonal circulation of RSV due to COVID-19, the dosing schedule can be extended beyond the five-dose regimen.
- In experts’ opinion, local surveillance data should guide the optimal timing of start month, duration of administration, and the number of doses.
|
|
Preterm infants without comorbidities
|
Early preterm infants (< 29 wGA): ≤ 12 months when the RSV season starts.
|
|
Moderate preterm infants (29–33 [290 to 326] wGA): ≤ 6 months at the beginning of the RSV season.
|
|
Late preterm infants (33–35 [330 to 350] wGA): ≤ 6 months when the RSV season begins or if they are born during the season and have any of the specified risk factors:
attending childcare,
permanently residing with children under five years old in the same household (including siblings),
being exposed to environmental air pollutants (smoking during pregnancy or in household).
|
|
Children with CLD/BPD
|
Palivizumab prophylaxis is recommended for all infants who are < 12 months of age.
≤ 24 months for those who continue to receive medications for CLD for at least six months from the start of the RSV season.
|
|
Children with CHD
|
Infants aged ≤ 12 months with hemodynamically significant-acyanotic CHD, who are:
receiving medications for congestive heart failure,
requiring cardiac surgery,
with severe pulmonary hypertension.
Infants aged ≤12 months with hemodynamically significant cyanotic CHD:
decisions regarding the administration of palivizumab prophylaxis should involve discussion with a pediatric cardiologist. These recommendations are applicable to eligible infants who are < 12 months of age the beginning of the RSV season.
Children aged 12–24 months who remain hemodynamically unstable and continue to take medication for cardiac conditions six months before the onset of epidemic season.
Following cardiopulmonary bypass surgery or extracorporeal membrane oxygenation (in children < 24 months of age), a single postoperative palivizumab dose (15 mg/kg) is recommended during the season, even when post-surgical defects are absent. The dose should be administered promptly once the infant is stable following the procedure, even if it is within a month of the previous dose, subsequent doses should be given on a monthly basis as scheduled.
Infants who are not considered at increased risk from RSV typically do not require immunoprophylaxis, including:
young children and infants with hemodynamically insignificant cardiac conditions (such as secundum atrial septal defect, small ventricular septal defect, pulmonary stenosis, uncomplicated aortic stenosis, mild coarctation of the aorta, and patent ductus arteriosus),
infants who have undergone corrective surgery, unless they still require medication for congestive heart failure,
infants not receiving medical treatment for mild cardiomyopathy,
children in their second year of age, unless otherwise specified.
|
|
Down's syndrome
|
Children with concomitant qualifying heart conditions, CLD, complications with airway clearance or born prematurely (prior to 350 wGA).
|
|
Cystic fibrosis
|
< 12 months for infants with CLD and/or nutritional deficiency.
< 24 months for children with severe lung conditions or weight for length below 10th percentile.
|
|
Anatomic pulmonary defects or neuromuscular disorder
|
< 12 months at the start of the RSV season.
|
|
Profoundly immunocompromised
|
< 24 months during the RSV season.
|
|
Multiple birth sets
|
If an infant at high risk of RSV is eligible for the season, the siblings from the same birth set also qualify for prophylaxis.
|
BPD: bronchopulmonary dysplasia; CHD: congenital heart disease; CLD: chronic lung disease; ICU: intensive care unit; RSV: respiratory syncytial virus;
wGA: weeks gestational age.
Barriers in utilization of palivizumab in RSV prophylaxis in the region
In the experts’ opinion, high cost of the prophylactic regimen was not a limiting factor for the GCC countries; several other factors such as the lack of centralized database or registry, poor compliance to the dosing regimen, lack of parental understanding on RSV burden and benefits of immunoprophylaxis, and cultural misbeliefs were the key obstacles to successful RSV immunoprophylaxis. Inaccurate use of terminology such as ‘immunization’ or ‘vaccination’ instead of ‘immunoprophylaxis’ is also known to contribute to non-adherence to the full dosing regimen.
The experts emphasized that compliance with dosing schedule, both in timing and frequency, is critical to achieving appropriate immunoprophylaxis efficacy. However, equating palivizumab to a vaccine may lead to false perceptions that a single injection may provide immunity against RSV infection. Non-adherence or deviation from the recommended dosing schedule may lower the efficacy of palivizumab and increase the risk of breakthrough RSV infection and hospitalization.68 Studies showed a significantly higher rate of RSV-related hospitalization in children who were noncompliant with monthly dosing of palivizumab prophylaxis.69,70
Expert recommendations to overcome barriers to RSV immunoprophylaxis
In light of these challenges, the expert panel members collectively shared several strategies aimed at enhancing compliance with RSV immunoprophylaxis in the region, including extensive parental counseling, dedicated monitoring teams focusing on high-risk patient population, frequent telephonic reminders to parents/caregivers before the appointment, and at-home or local administration. Using these interventions, a study from Qatar reported significant improvement in the compliance rate over the three successive RSV seasons (2009–2012) from 57.7% to 94.2% (p < 0.05). Improved compliance with palivizumab administration resulted in a decline in hospitalization rate from 3.7% to 1.7%.20 Another hospital-based study from Dubai (925 children enrolled over five RSV seasons) reported a considerable reduction in RSV-related hospitalization rate from 9.23% in 2012–2013 to 0.67% in 2016–2017, attributed to a high compliance rate of 90.9% over the study period.19 Such findings underscore the critical importance of compliance in achieving effective outcomes with palivizumab in RSV prophylaxis.
Parental beliefs or perception regarding immunoprophylaxis is essential while designing interventional strategies to aid compliance. Therefore, it is critically important that the parents/caregivers are empowered with a clear understanding of RSV burden, the potential risk of long-term respiratory morbidity in severe cases, and the importance of palivizumab prophylaxis. Experts also emphasized on the importance of appropriate terminology to aid compliance; HCPs should be encouraged to use the term ‘immunoprophylaxis’ rather than ‘vaccine’ to convey its purpose and potential benefits accurately.
Digital referral forms incorporating user-intuitive instructions may aid HCPs and nurses in streamlining the referral process and facilitate expeditious patient enrollment. Active follow-up of the cases, particularly those presented at the emergency department is imperative to ascertain reasons for any missed referrals. It is recommended to establish and maintain a database to facilitate dosing schedule, dispatch timely reminders, and contact eligible candidates for subsequent seasons. To prepare for an upcoming season, drug supplies can be procured in advance and an announcement can be disseminated regarding the eligibility criteria.
More importantly, comprehensive analysis of regional data and establishing local registries may help yield valuable insights into region-specific sociodemographic risk factors, which may be beneficial for evaluating the need for potential revisions in the recommendations for RSV prophylaxis. For RSV surveillance, multiplex polymerase chain reaction and antigen assays can be performed on patients hospitalized with respiratory infections during outpatient department visits. Seasonality onset may be delineated by analyzing laboratory reports and employing clinical criteria, particularly focusing on the pattern of admissions among high-risk demographic groups. In the UAE, the main microbiology laboratory disseminates an official announcement at the onset of RSV season, which is defined by either a mean RSV positivity rate of > 3% as determined by polymerase chain reaction analysis or occurrence of two or more RSV-related hospitalizations for two consecutive weeks. Additionally, the RSV Hospitalization Surveillance Network interactive dashboards can be used to monitor real-time trends of laboratory-confirmed RSV-related hospitalizations and allow comparison among different demographic population groups and across seasons. Figure 2 provides an overview of the region-specific barriers to RSV immunoprophylaxis and their expert-recommended solutions.
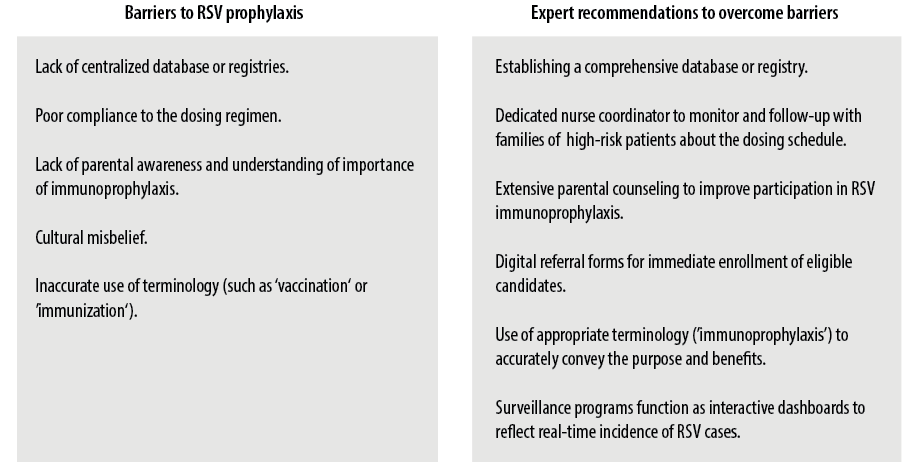 Figure 2: Expert recommendations to overcome the barriers to respiratory syncytial virus (RSV) immunoprophylaxis.
Figure 2: Expert recommendations to overcome the barriers to respiratory syncytial virus (RSV) immunoprophylaxis.
Conclusion
The RSV remains the leading cause of LRTIs among young children in the GCC region. Hospitalization and morbidity caused by RSV have a substantial impact on high-risk children and their families. More than the financial burden, the lack of a centralized database, poor compliance with the dosing regimen, parental ignorance, and cultural misbeliefs, act as significant regional barriers to overcoming the challenges posed by the disease and its consequences. Expert recommendations to reinforce palivizumab prophylaxis include establishing comprehensive databases or local registries, extensive parental counselling, and dedicated monitoring of high-risk population groups. These recommendations may serve as a reference guide for HCPs, RSV program leaders, and those involved in enrolling eligible patients in preventive RSV programs. Moreover, recent advances in RSV prevention strategies such as longer-acting mAbs and vaccines targeting infants, pregnant women, and adults offer significant promise for the near future.
Disclosure
The authors declare no conflict of interest and financial disclosure. AstraZeneca FZ LLC funds medical writing assistance for this manuscript.
Acknowledgments
The authors would like to thank Parul Rishi and Dr. Suvarna Chavan of Fortrea Scientific Pvt. Ltd. for medical writing support in accordance with Good Publication Practice 2022 guidelines.
references
- 1. Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al; Respiratory Virus Global Epidemiology Network; RESCEU investigators. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022 May;399(10340):2047-2064.
- 2. Fauroux B, Simões EA, Checchia PA, Paes B, Figueras-Aloy J, Manzoni P, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther 2017 Jun;6(2):173-197.
- 3. Chatterjee A, Mavunda K, Krilov LR. Current state of respiratory syncytial virus disease and management. Infect Dis Ther 2021 Mar;10(Suppl 1):5-16.
- 4. Havdal LB, Bøås H, Bekkevold T, Bakken Kran AM, Rojahn AE, Størdal K, et al. Risk factors associated with severe disease in respiratory syncytial virus infected children under 5 years of age. Front Pediatr 2022 Aug;10:1004739.
- 5. Shi T, Vennard S, Mahdy S, Nair H; RESCEU investigators. Risk factors for poor outcome or death in young children with respiratory syncytial virus-associated acute lower respiratory tract infection: a systematic review and meta-analysis. J Infect Dis 2022 Aug;226(Suppl 1):S10-S16.
- 6. Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, Rodríguez-Tenreiro C, Sly P, Ramilo O, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis 2018 Apr;217(9):1356-1364.
- 7. Alkharsah KR. The scope of respiratory syncytial virus infection in a tertiary hospital in the eastern province of Saudi Arabia and the change in seasonal pattern during and after the COVID-19 pandemic. Medicina (Kaunas) 2022 Nov;58(11):1623.
- 8. Yassine HM, Sohail MU, Younes N, Nasrallah GK. Systematic review of the respiratory syncytial virus (RSV) prevalence, genotype distribution, and seasonality in children from the Middle East and North Africa (MENA) region. Microorganisms 2020 May;8(5):713.
- 9. Ahmed A, Parveen S, Al-Hassinah SM, Al-Amery SF. An overview of respiratory syncytial virus infections in Saudi Arabia. J Infect Dev Ctries 2018 Nov;12(11):929-936.
- 10. Bukhari EE, Elhazmi MM. Viral agents causing acute lower respiratory tract infections in hospitalized children at a tertiary care center in Saudi Arabia. Saudi Med J 2013 Nov;34(11):1151-1155.
- 11. Khamis FA, Al-Kobaisi MF, Al-Areimi WS, Al-Kindi H, Al-Zakwani I. Epidemiology of respiratory virus infections among infants and young children admitted to hospital in Oman. J Med Virol 2012 Aug;84(8):1323-1329.
- 12. Mohamed AM, Al Sayyad A, Matar E, Isa HM, Hasan WF, Hashim NS, et al. Factors associated with poor outcomes in patients with severe acute respiratory infections in Bahrain. Influenza Other Respir Viruses 2023 Apr;17(4):e13133.
- 13. Huang K, Incognito L, Cheng X, Ulbrandt ND, Wu H. Respiratory syncytial virus-neutralizing monoclonal antibodies motavizumab and palivizumab inhibit fusion. J Virol 2010 Aug;84(16):8132-8140.
- 14. BeyfortusTM (nirsevimab-alip) injection. FDA label. 2023 [cited 2023 November 7]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761328s000lbl.pdf.
- 15. Alharbi AS, Alqwaiee M, Al-Hindi MY, Mosalli R, Al-Shamrani A, Alharbi S, et al. Bronchiolitis in children: The Saudi initiative of bronchiolitis diagnosis, management, and prevention (SIBRO). Ann Thorac Med 2018;13(3):127-143.
- 16. Dubai standards of care – 2019. Respiratory syncytial virus (RSV) immunoprophylaxis. 2019 [cited 2023 August 1]. Available from: https://www.isahd.ae/content/docs/DSC%20RSV.pdf.
- 17. American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014 Aug;134(2):e620-e638.
- 18. Al Harbi AS. War against respiratory syncytial virus. An 8-year experience at a tertiary hospital. Saudi Med J 2018 Dec;39(12):1200-1206.
- 19. Elhalik M, El-Atawi K, Dash SK, Faquih A, Satyan AD, Gourshettiwar N, et al. Palivizumab prophylaxis among infants at increased risk of hospitalization due to respiratory syncytial virus infection in UAE: a hospital-based study. Can Respir J 2019 Dec;2019:2986286.
- 20. Abushahin A, Janahi I, Tuffaha A. Effectiveness of palivizumab immunoprophylaxis in preterm infants against respiratory syncytial virus disease in Qatar. Int J Gen Med 2018 Jan;11:41-46.
- 21. Farrag MA, Hamed ME, Amer HM, Almajhdi FN. Epidemiology of respiratory viruses in Saudi Arabia: toward a complete picture. Arch Virol 2019 Aug;164(8):1981-1996.
- 22. A Al-Sharif H, El-Kafrawy SA, Yousef JM, Kumosani TA, Kamal MA, Khathlan NA, et al. Dominance of the ON1 genotype of RSV-A and BA9 genotype of RSV-B in respiratory cases from Jeddah, Saudi Arabia. Genes (Basel) 2020 Nov;11(11):1323.
- 23. Madi N, Chehadeh W, Asadzadeh M, Al-Turab M, Al-Adwani A. Analysis of genetic variability of respiratory syncytial virus groups A and B in Kuwait. Arch Virol 2018 Sep;163(9):2405-2413.
- 24. Packnett ER, Winer IH, Oladapo A, Wojdyla M. Risk of RSV-related hospitalization is associated with gestational age in preterm (born at 29-34 wGA) infants without outpatient palivizumab administration. Hum Vaccin Immunother 2023 Aug;19(2):2252289.
- 25. McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol 2016 Nov;36(11):990-996.
- 26. Simões EA, Bont L, Manzoni P, Fauroux B, Paes B, Figueras-Aloy J, et al. Past, present and future approaches to the prevention and treatment of respiratory syncytial virus infection in children. Infect Dis Ther 2018 Mar;7(1):87-120.
- 27. Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 2013 Apr;(4):CD006602.
- 28. Wang DY, Li A, Paes B, Mitchell I, Lanctôt KL; CARESS Investigators. First versus second year respiratory syncytial virus prophylaxis in chronic lung disease (2005-2015). Eur J Pediatr 2017 Mar;176(3):413-422.
- 29. Paes B, Carbonell-Estrany X. Respiratory syncytial virus prophylaxis for children with chronic lung disease: have we got the criteria right? Expert Rev Anti Infect Ther 2019 Apr;17(4):211-222.
- 30. Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH Jr, et al; Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003 Oct;143(4):532-540.
- 31. Chang RK, Chen AY. Impact of palivizumab on RSV hospitalizations for children with hemodynamically significant congenital heart disease. Pediatr Cardiol 2010 Jan;31(1):90-95.
- 32. Chiu SN, Wang JN, Fu YC, Chung HT, Chang LY, Wu MH, et al. Efficacy of a novel palivizumab prophylaxis protocol for respiratory syncytial virus infection in congenital heart disease: a multicenter study. J Pediatr 2018 Apr;195:108-114.e1.
- 33. Mohammed MH, Agouba R, Obaidy IE, Alhabshan F, Abu-Sulaiman R. Palivizumab prophylaxis against respiratory syncytial virus infection in patients younger than 2 years of age with congenital heart disease. Ann Saudi Med 2021;41(1):31-35.
- 34. Li A, Wang DY, Lanctôt KL, Mitchell I, Paes BA; CARESS Investigators. CARESS Investigators. Comparing first- and second-year palivizumab prophylaxis in patients with hemodynamically significant congenital heart disease in the CARESS database (2005-2015). Pediatr Infect Dis J 2017 May;36(5):445-450.
- 35. Paes B, Mitchell I, Yi H, Li A, Lanctôt KL; CARESS Investigators. Hospitalization for respiratory syncytial virus illness in Down syndrome following prophylaxis with palivizumab. Pediatr Infect Dis J 2014 Feb;33(2):e29-e33.
- 36. Kimura T, Takeuchi M, Kawakami K. Utilization and efficacy of palivizumab for children with Down syndrome. Pediatr Int 2020 Jun;62(6):677-682.
- 37. Kua KP, Lee SW. Systematic review of the safety and efficacy of palivizumab among infants and young children with cystic fibrosis. Pharmacotherapy 2017 Jun;37(6):755-769.
- 38. Sánchez-Solis M, Gartner S, Bosch-Gimenez V, Garcia-Marcos L. Is palivizumab effective as a prophylaxis of respiratory syncytial virus infections in cystic fibrosis patients? A meta-analysis. Allergol Immunopathol (Madr) 2015;43(3):298-303.
- 39. Fink AK, Graff G, Byington CL, Loeffler DR, Rosenfeld M, Saiman L. Palivizumab and long-term outcomes in cystic fibrosis. Pediatrics 2019 Jul;144(1):e20183495.
- 40. Teusink-Cross A, Davies SM, Danziger-Isakov L, El-Bietar J, Grimley MS. Restrictive palivizumab use does not lead to increased morbidity and mortality in pediatric hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant 2016 Oct;22(10):1904-1906.
- 41. Committee on Infectious Diseases. From the American Academy of Pediatrics: Policy statements–Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics 2009 Dec;124(6):1694-1701.
- 42. Anderson EJ, DeVincenzo JP, Simões EA, Krilov LR, Forbes ML, Pannaraj PS, et al. SENTINEL1: two-season study of respiratory syncytial virus hospitalizations among U.S. infants born at 29 to 35 weeks’ gestational age not receiving immunoprophylaxis. Am J Perinatol 2020 Mar;37(4):421-429.
- 43. Rajah B, Sánchez PJ, Garcia-Maurino C, Leber A, Ramilo O, Mejias A. Impact of the updated guidance for palivizumab prophylaxis against respiratory syncytial virus infection: a single center experience. J Pediatr 2017 Feb;181:183-188.e1.
- 44. Capizzi A, Silvestri M, Orsi A, Cutrera R, Rossi GA, Sacco O. The impact of the recent AAP changes in palivizumab authorization on RSV-induced bronchiolitis severity and incidence. Ital J Pediatr 2017 Aug;43(1):71.
- 45. Luna MS, Muñuzuri AP, Castellanos JL, Campillo CW, López ES, Fernández IB, et al. [An update of the recommendations of the Spanish Neonatology Society for the use of palivizumab as prophylaxis for severe infections due to syncytial respiratory virus in high risk infants]. An Pediatr (Engl Ed) 2019;91(5):348-350.
- 46. Bollani L, Baraldi E, Chirico G, Dotta A, Lanari M, Del Vecchio A, et al; Italian Society of Neonatology. Revised recommendations concerning palivizumab prophylaxis for respiratory syncytial virus (RSV). Ital J Pediatr 2015 Dec;41:97.
- 47. Sampalis JS, Langley J, Carbonell-Estrany X, Paes B, O’Brien K, Allen U, et al. Development and validation of a risk scoring tool to predict respiratory syncytial virus hospitalization in premature infants born at 33 through 35 completed weeks of gestation. Med Decis Making 2008;28(4):471-480.
- 48. Blanken MO, Koffijberg H, Nibbelke EE, Rovers MM, Bont L, Dutch RS, et al; Dutch RSV Neonatal Network. Prospective validation of a prognostic model for respiratory syncytial virus bronchiolitis in late preterm infants: a multicenter birth cohort study. PLoS One 2013;8(3):e59161.
- 49. Blanken MO, Paes B, Anderson EJ, Lanari M, Sheridan-Pereira M, Buchan S, et al. Risk scoring tool to predict respiratory syncytial virus hospitalisation in premature infants. Pediatr Pulmonol 2018 May;53(5):605-612.
- 50. Paes B, Fullarton JR, Rodgers-Gray BS, Carbonell-Estrany X. Adoption in Canada of an international risk scoring tool to predict respiratory syncytial virus hospitalization in moderate-to-late preterm infants. Curr Med Res Opin 2021 Jul;37(7):1149-1153.
- 51. Butt M, Elliott L, Guy F, Symington A, Paes B. Comparison of the Canadian vs. the international risk scoring tool for respiratory syncytial virus prophylaxis in moderate-to-late preterm infants. Front Pediatr 2023 Jan;10:997349.
- 52. Rodgers-Gray BS, Fullarton JR, Carbonell-Estrany X, Keary IP, Tarride JÉ, Paes BA. Impact of using the International Risk Scoring Tool on the cost-utility of palivizumab for preventing severe respiratory syncytial virus infection in Canadian moderate-to-late preterm infants. J Med Econ 2023;26(1):630-643.
- 53. Wang X, Li Y, Nair H, Campbell H; RESCEU Investigators. RESCEU Investigators. Time-varying association between severe respiratory syncytial virus infections and subsequent severe asthma and wheeze and influences of age at the infection. J Infect Dis 2022 Aug;226(Suppl 1):S38-S44.
- 54. Shi T, Ooi Y, Zaw EM, Utjesanovic N, Campbell H, Cunningham S, et al; RESCEU Investigators. Association between respiratory syncytial virus-associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J Infect Dis 2020 Oct;222(Suppl 7):S628-S633.
- 55. Pokrzywinski RM, Swett LL, Pannaraj PS, Yi J, Pavilack MS, Kumar VR, et al. Impact of respiratory syncytial virus-confirmed hospitalizations on caregivers of US preterm infants. Clin Pediatr (Phila) 2019 Jul;58(8):837-850.
- 56. Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, et al; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013 May;368(19):1791-1799.
- 57. Scheltema NM, Nibbelke EE, Pouw J, Blanken MO, Rovers MM, Naaktgeboren CA, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respir Med 2018 Apr;6(4):257-264.
- 58. Yoshihara S, Kusuda S, Mochizuki H, Okada K, Nishima S, Simões EA; C-CREW Investigators. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics 2013 Nov;132(5):811-818.
- 59. Weinberger Opek M, Yeshayahu Y, Glatman-Freedman A, Kaufman Z, Sorek N, Brosh-Nissimov T. Delayed respiratory syncytial virus epidemic in children after relaxation of COVID-19 physical distancing measures, Ashdod, Israel, 2021. Euro Surveill 2021 Jul;26(29):2100706.
- 60. Pérez-López A, Al Mana H, Iqbal M, Suleiman M, Hasan MR, Tang P. Variations in respiratory syncytial virus activity following the relaxation of COVID-19 restrictions in Qatar. J Travel Med 2022 Sep;29(6):taac065.
- 61. American Academy of Pediatrics. Updated guidance: use of palivizumab prophylaxis to prevent hospitalization from severe respiratory syncytial virus infection during the 2022-2023 RSV season. 2022 [cited 2023 June 14]. Available from: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-for-use-of-palivizumab-prophylaxis-to-prevent-hospitalization/.
- 62. COVID-19 rapid policy statement. Palivizumab passive immunisation against respiratory syncytial virus (RSV) in at risk pre-term infants. [cited 2023 July 20]. Available from: https://www.england.nhs.uk/south/wp-content/uploads/sites/6/2021/07/phe-ref-ra-21.035-palivizumab-passive-immunisation-against-respiratory-syncytial-virus-rsv-in-at-risk-pre-term.pdf.
- 63. Alharbi AS, Alzahrani M, Alodayani AN, Alhindi MY, Alharbi S, Alnemri A. Saudi experts’ recommendation for RSV prophylaxis in the era of COVID-19: consensus from the Saudi Pediatric Pulmonology Association. Saudi Med J 2021 Apr;42(4):355-362.
- 64. Al-Alaiyan S, Pollack P, Notario GF. Safety and pharmacokinetics of extended use of palivizumab in Saudi Arabian infants and children. Drugs Context 2015 Feb;4:212270.
- 65. Synagis (Palivizumab) solution for injection. Summary of product characteristics. [cited 2023 March 5]. Available from: https://www.ema.europa.eu/en/documents/product-information/synagis-epar-product-information_en.pdf.
- 66. Esposito S, Abu-Raya B, Bonanni P, Cahn-Sellem F, Flanagan KL, Martinon Torres F, et al. Coadministration of anti-viral monoclonal antibodies with routine pediatric vaccines and implications for nirsevimab use: a white paper. Front Immunol 2021 Aug;12:708939.
- 67. CDC. Timing and spacing guidelines for immunization. 2023 [cited 2023 November 28]. Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/timing.html.
- 68. Wong SK, Li A, Lanctôt KL, Paes B. Adherence and outcomes: a systematic review of palivizumab utilization. Expert Rev Respir Med 2018 Jan;12(1):27-42.
- 69. Stewart DL, Ryan KJ, Seare JG, Pinsky B, Becker L, Frogel M. Association of RSV-related hospitalization and non-compliance with palivizumab among commercially insured infants: a retrospective claims analysis. BMC Infect Dis 2013 Jul;13(1):334.
- 70. Torchin H, Charkaluk ML, Rousseau J, Marchand-Martin L, Treluyer L, Nuytten A, et al. Full compliance with respiratory syncytial virus prophylaxis was associated with fewer respiratory-related hospital admissions in preterm children: a cohort study. Acta Paediatr 2021 May;110(5):1633-1638.