Pulmonary vein thrombosis (PVT) is an uncommon and potentially life-threatening condition often overlooked in clinical practice, posing a significant threat.1 Onuigbo’s autopsy series emphasizes the importance of increased awareness, drawing attention to the historical underdetection of PVT.1 The true incidence of PVT is still difficult to ascertain and primarily depends on case reports found in the literature. PVT is uncommon because of a strong network of venous collateral vessels that drain the lung, yet obstructive episodes can occur in certain clinical circumstances.2
PVT has been reported in several clinical settings, including lung transplants, post-lobectomy cases, and in association with metastatic cancer. Some cases are classified as idiopathic. Recognizing these associations is crucial for a comprehensive understanding.3,4 Symptoms such as dyspnea, cough, or hemoptysis, are nonspecific, making diagnosis challenging. Accurate diagnosis requires high suspicion, as the condition can be easily missed. Failure to identify and treat PVT in a timely manner may lead to catastrophic consequences, including peripheral embolization and acute stroke. Given its rarity and severe complications, a comprehensive review of the literature is essential to establish a foundational understanding of PVT. This review provides critical background information for future research and clinical awareness.
Clinical presentation
Patients with PVT often present with no apparent symptoms or with nonspecific indications such as cough, hemoptysis, and dyspnea, which are commonly attributed to pulmonary edema or infarction.1–8 The hemodynamic manifestations of PVT lack specificity and can mimic conditions such as acute graft rejection, presenting with hypoxemia and interstitial infiltrates in the transplanted lung. They may also resemble symptoms of right ventricular failure or reperfusion injury.9
In most PVT cases, there is a simultaneous elevation in pulmonary artery pressure, systemic hypotension, and diminished cardiac output. Respiratory parameters decline, with reduced oxygenation, increased carbon dioxide levels (hypercapnia), and decreased pulmonary compliance.10
It is noteworthy that the clinical presentation of PVT can vary. For example, in one case, persistent fever was the sole symptom.11 Schiller and Madge noted systolic murmurs in various locations in three out of 16 patients, though the significance of this observation remains unclear.1 Neurologic deficits resulting from cerebral emboli may manifest as the only symptom in certain cases.5,12
This diverse array of symptoms and their varying severity underscores the complexity of diagnosing PVT. Healthcare professionals must recognize these varied presentations to enhance diagnostic accuracy and ensure timely intervention.
Risk factors
Case reports highlighted various risk factors associated with PVT, as illustrated in Figure 1. These included vein-related surgical procedures such as lobectomies or lung transplants, atrial fibrillation treated with radiofrequency catheter ablation, sclerosing mediastinitis, and certain primary or secondary lung tumors. Atrial myxoma, congenital pulmonary venous stenosis, and mitral stenosis with an obstructive left atrial clot are other less common risk factors.2
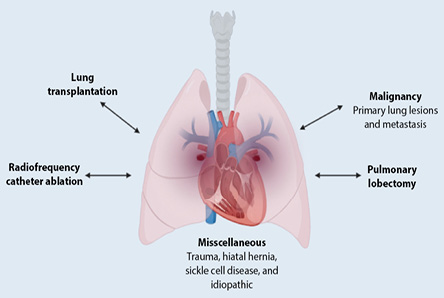 Figure 1: Pulmonary vein thrombosis risk factors.
Figure 1: Pulmonary vein thrombosis risk factors.
The earliest documented case of non-surgical PVT, dating back to 1925, involved the autopsy of a young male with testicular cancer who tragically succumbed to an acute collapse during hospitalization.13
Malignancy
The predominant malignant trigger for PVT is a primary lung neoplasm, particularly bronchogenic carcinoma.1 However, PVT can also occur after metastatic tumors,14 such as liposarcoma,14 small cell lung cancer,15 mantle cell lymphoma (which starts in the small intestine),6 and metastatic sarcoma.6 Predisposing factors include stagnation from mechanical compression related to tumor size and a paraneoplastic hypercoagulable state. Damage to the endothelium may result from this compression.16
Atrial fibrillation
Uncertainty persists regarding whether atrial fibrillation, leading to blood stasis, constitutes a risk factor for thrombus development.17 There is also a lack of clarity concerning the thromboembolic risk associated with left upper pulmonary vein thrombus in atrial fibrillation, particularly in the post-cardioversion period. Given the increasing prevalence of atrial fibrillation, catheter procedures for ablation have emerged as a novel treatment for drug-refractory cases, but complications from radiofrequency ablation can significantly affect the pulmonary system.
Various studies have reported a wide range in the prevalence of pulmonary vein stenosis following radiofrequency ablation, ranging from 3% to 42%, depending on the assessment method and technique used. In the largest available consecutive series, severe pulmonary vein stenosis—defined as a 70% luminal narrowing—was observed in 5% of 335 patients, with 2.1% experiencing total occlusion of at least one pulmonary vein.18 Another series documented total pulmonary vein occlusion in 1.3% of 229 patients.18 Additionally, cases of severe pulmonary hypertension have been reported in patients who developed significant narrowing of all four pulmonary veins near the left atrial junction three months after undergoing successful radiofrequency ablation.18 López-Reyes et al,19 reported another case of PVT post radiofrequency ablation of the pulmonary veins, where pulmonary resection was performed due to hemorrhagic recurrence.
Post-lung transplantation
PVT has been reported in up to 15% of lung transplant patients within the first 48 hours, with the risk lasting up to two years.6,7,13,16 Although improved surgical techniques have reduced the incidence,4 PVT in transplant patients can lead to pulmonary venous flow obstruction and thromboembolic events. Thrombus formation is often linked to endothelial injury during surgery and blood stasis in the pulmonary vein stump.16
Interestingly, no specific risk factors have been identified for donors or recipients, such as a history of deep vein thrombosis or prothrombotic disorders.4,20 Some patients received perioperative bovine pancreatic trypsin inhibitor (aprotinin), and the thrombotic risk associated with this agent remains unclear.20 An analysis of 153 lung transplant recipients revealed that 45 patients developed upper or lower extremity deep vein thrombosis. This occurrence showed a strong association with factors such as the presence of an indwelling catheter, infections, and the use of prednisone with mycophenolate mofetil plus either cyclosporine or tacrolimus.20
Post-lobectomy
There have been only six documented instances of PVT following lobectomy.21–25 In all reported cases, the involvement was exclusive to the left upper lobe resection, resulting in thrombus formation within the left upper pulmonary vein stump. The actual frequency of PVT occurrences post-lobectomy remains elusive and likely underestimated, particularly among asymptomatic individuals who forego postoperative imaging using transesophageal echocardiography (TEE) or computed tomography.16 Despite its potential underdiagnosis, PVT after lobectomy is not benign, posing risks of thromboembolic complications, including transient ischemic attacks and strokes.
Ohtaka et al,17 conducted a retrospective study, revealing that thrombosis in the pulmonary vein stump occurred in 3.3% of patients who underwent lobectomy and 17.9% of those who specifically underwent left upper lobectomy. Clinically, thrombi in the systemic circulation usually arise from atrial fibrillation. While left lobectomy is suggested as a potential risk factor for atrial fibrillation, 18 patients in the Ohtaka study exhibited no signs of atrial fibrillation when the thrombosis was diagnosed.17 This underscores the unique association of PVT with left upper lobe resection, emphasizing the importance of vigilant monitoring and thorough diagnostic approaches in such cases.
Others
PVT incidents have been sporadically documented in cases of blunt chest trauma and sickle cell disease,5,26 where vascular stasis due to hypoxia and sickling was hypothesized as the underlying cause.16 Another reported case identified a large hiatal hernia as a potential contributor to PVT, attributing it to compression of intrathoracic structures.5
In the realm of idiopathic causes, PVT has been associated with hemoglobinopathy.7,27 Remarkably, only two previous instances of spontaneous idiopathic PVT have been documented.28,29
Diagnosis
Clinical diagnosis of PVT poses challenges due to nonspecific symptoms. Previous cases have relied on either pulmonary angiogram or pathological examination for accurate diagnosis.3 The discovery of unsuspected PVT during surgery carries a grim prognosis due to the high likelihood of massive embolization. Therefore, preoperative diagnosis becomes crucial, with alternative techniques of pulmonary venous clamping and cardiopulmonary bypass being potential strategies to minimize the risk of embolization.1
Postoperative diagnosis of PVT after lobectomy or lung transplantation does not typically present specific difficulties. It often manifests a few days post-surgery with sudden-onset dyspnea, and chest X-ray studies revealed unilateral airspace disease without volume loss.5 Notably, physical examination and plain chest radiography do not aid diagnosis.4 Identifying PVT requires a comprehensive approach involving conventional imaging modalities such as pulmonary angiography, transthoracic echocardiography, and TEE, which can differentiate between tumor and thrombus. Additionally, late-phase computed tomography with intravenous contrast material injection is employed to reduce flow artifacts. Magnetic resonance imaging has also emerged as a diagnostic tool.1,2,7,16
Complications
Complications of PVT include pulmonary infarction, pulmonary edema, right ventricular failure, and allograft failure [Figure 2].4,30 Despite being less frequently documented, peripheral embolism poses a potential complication, leading to incidents of limb ischemia, stroke, and renal infarction.31–34
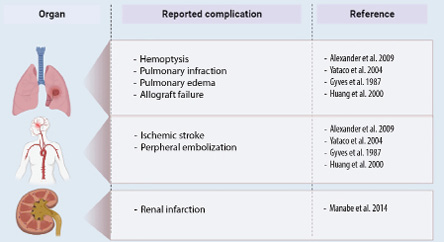 Figure 2: Complications of pulmonary vein thrombosis.
Figure 2: Complications of pulmonary vein thrombosis.
In lung transplant patients, the occurrence of acute PVT can have catastrophic consequences, leading to early allograft failure. This failure is attributed to the obstruction of pulmonary venous flow, causing severe pulmonary edema.6,10 A prospective study involving 87 consecutive adult lung transplant recipients utilized TEE within two days after surgery. During this assessment, PVT was diagnosed in 13 (15%) patients; five (38%) of these patients succumbed during the perioperative period, with three of the five deaths resulting from graft failure. Another report highlighted a 90-day mortality rate of 38% following lung transplant.35
Moreover, PVT has been observed to lead to systemic emboli, resulting in cerebrovascular accidents.1,6,20,21,23,27 A thorough examination of existing literature reveals a limited number of case reports emphasizing the occurrence of stroke and systemic embolization attributed to PVT. To date, no randomized control trials have been conducted on this specific aspect. In a 2002 study conducted by Grau et al,33 patients with cryptogenic stroke were scrutinized for PVT using magnetic resonance venography, and the study did not establish PVT as a significant contributor to the etiology of ischemic stroke in these cases. However, the study faced notable limitations, primarily due to frequent inadequate visualization of the left pulmonary veins, stemming from constraints in magnetic resonance imaging techniques. With advancements in radiologic techniques over the last 10–15 years, it is conceivable that more cases of PVT, particularly within the left pulmonary venous system, could be identified.33
Furthermore, renal infarction has been identified as a complication associated with PVT. To our knowledge, only six instances of renal infarction following lung resections have been documented, and none of these cases exhibited detectable, well-known causes for renal infarction.34 Among the two patients who experienced renal infarction, PVT was visually confirmed. In the remaining four cases of early postoperative renal infarction, the diagnosis was established as idiopathic renal infarction, potentially linked to the postoperative and/or paraneoplastic hypercoagulable state. However, in three cases, the coexistence of thrombus in the pulmonary vein was not examined. In one case, it was not mentioned, as PVT had not been previously recognized as a risk factor for renal infarction. Furthermore, the frequency of thrombus formation in the stump of the left superior pulmonary vein is more prevalent than previously assumed. Therefore, the history of left upper lobectomy should be considered a potential risk factor for renal infarction, and PVT must be recognized as a potential source of thromboembolism to accurately diagnose the cause of renal infarction.36
In addition, other rare complications commonly associated with idiopathic PVT encompass pulmonary gangrene, peripheral embolism, and massive hemoptysis.7,27,37
Management
The management of PVT should be put in the context of the underlying disease pathology.36 At present, there are no randomized controlled trials, evidence-based guidelines, or expert consensus recommendations for PVT management. The management may include the use of antibiotics, systemic anticoagulation, thrombectomy, and/or lung resection.36
Antibiotics therapy
Post-lung transplant or lobectomy patients are frequently initiated on antibiotics due to an increased infection risk.20,36
However, no specific studies recommend which antibiotics to use in PVT cases, leaving this decision to clinical judgment based on the patient’s overall condition. Several factors should be considered when selecting an antibiotic regimen: the patient’s age, immune status, the specific lung procedure performed, and the overall severity of the patient’s illness.
Immunocompromised patients, particularly those post-lung transplant, may require broader-spectrum antibiotics or prophylaxis against opportunistic infections. The type of lung procedure (e.g., transplant vs. resection) can influence the risk of specific infections. Additionally, critically ill patients may necessitate initial broad-spectrum antibiotic coverage, with adjustments based on clinical response and culture results.
Despite these considerations, there is no evidence favoring any specific antibiotic regimen for treating or preventing thrombosis in PVT. Moreover, no studies have compared outcomes between using antibiotics versus not using antibiotics in patients with PVT following lung transplant or resection. Therefore, the use of antibiotics in this context remains empirical, guided by clinical judgment, patient health, and any present signs or risk factors for infection.
Anticoagulation
In a systematic review of 28 case reports, 23 cases were treated with anticoagulation.38 The anticoagulation management of PVT in malignancy should fall under the umbrella of cancer-associated thrombosis (CAT). There are several evidence-based guidelines that address CAT.38–40 In general, anticoagulation is usually initiated with either low molecular weight heparin (LMWH) or unfractionated heparin depending on the renal function and bleeding risk. LMWH used to be the standard care of long-term management of CAT, however, there is now enough data to show the non-inferiority of direct oral anticoagulant (DOAC) therapy.39–41
There are several case reports of successful treatment of PVT using DOACs with different underlying pathologies.42–45 DOACs have been shown to be non-inferior to warfarin or LMWH in several clinical trials in the context of venous thromboembolism, CAT, and stroke prevention in atrial fibrillation.46–49 In addition, DOACs are associated with lower major bleeding risk, do not require monitoring and are more convenient. Therefore, DOACs are the preferred oral anticoagulation option in patients with PVT, in the right context.
The duration of anticoagulation in PVT also depends on the clinical context. For example, in the context of CAT, PVT should be treated for at least six months.37–39 Regardless of the underlying pathology, anticoagulation should be given for at least 3–6 months.36
Embolectomy and lung resection
Successful embolectomy was reported in two case reports. The first case was in the context of the progression of PVT despite being on anticoagulation, and the other was after a bilateral lung transplant.9,38 Lung resection might be indicated in cases with massive hemoptysis, pulmonary necrosis, or gangrene.8,36
Thrombolysis
Thrombolysis with recombinant tissue plasminogen activator has been used successfully in a single case report of a lung transplant recipient with a left atrial thrombus.50 However, this remains anecdotal and not broadly recommended.
Conclusion
Management of PVT should be tailored to the underlying pathology, with options including antibiotics, anticoagulation, thrombectomy, and lung resection. Antibiotics are essential following lung transplant or resection because of the risk of infection, despite the lack of randomized controlled studies. Anticoagulation, particularly with DOACs, is commonly used, offering convenience and lower bleeding risks. Anticoagulation therapy generally spans 3–6 months, contingent on the clinical scenario. In specific cases, embolectomy or lung resection may be necessary. Further research is essential to establish comprehensive guidelines for PVT management.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors used Quillbot to improve the language and eliminate grammatical mistakes. Then, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
references
- 1. Kim NH, Roldan CA, Shively BK. Pulmonary vein thrombosis. Chest 1993 Aug;104(2):624-626.
- 2. Cavaco RA, Kaul S, Chapman T, Casaretti R, Philips B, Rhodes A, et al. Idiopathic pulmonary fibrosis associated with pulmonary vein thrombosis: a case report. Cases J 2009 Dec;2:9156.
- 3. Garcia MJ, Rodriguez L, Vandervoort P. Pulmonary vein thrombosis and peripheral embolization. Chest 1996 Mar;109(3):846-847.
- 4. Saoraya J, Inboriboon PC. Pulmonary vein thrombosis associated with a large hiatal hernia. J Emerg Med 2013 Mar;44(3):e299-e301.
- 5. Mumoli N, Cei M. Idiopathic pulmonary vein thrombosis. J Emerg Med 2012 Feb;42(2):182-183.
- 6. Akiode O, Prakash G. Pulmonary vein thrombosis associated with metastatic carcinoma. Fed Pract 2014;31(12):26-28.
- 7. Porres DV, Morenza OP, Pallisa E, Roque A, Andreu J, Martínez M. Learning from the pulmonary veins. Radiographics 2013;33(4):999-1022.
- 8. Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax 2003 Jun;58(6):550-551.
- 9. Nagahiro I, Horton M, Wilson M, Bennetts J, Spratt P, Glanville AR. Pulmonary vein thrombosis treated successfully by thrombectomy after bilateral sequential lung transplantation: report of a case. Surg Today 2003;33(4):282-284.
- 10. Cywinski JB, Wallace L, Parker BM. Pulmonary vein thrombosis after sequential double-lung transplantation. J Cardiothorac Vasc Anesth 2005 Apr;19(2):225-227.
- 11. Gyves-Ray KM, Spizarny DL, Gross BH. Unilateral pulmonary edema due to postlobectomy pulmonary vein thrombosis. AJR Am J Roentgenol 1987 Jun;148(6):1079-1080.
- 12. Stang MR, Hinderliter AL, Gott KK, Paradowski LJ, Aris RM. Atrial anastomotic thrombus causes neurologic deficits in a lung transplant recipient. Transplantation 1996 Sep;62(5):693-695.
- 13. Webb-Johnson AE. Embryoma of the testis: sudden death from thrombosis of pulmonary veins. Br Med J 1925 Dec;2(3388):1048-1049.
- 14. Tamizifar B, Zadeh MR, Foroghi E. Pulmonary vein thrombosis after metastatic liposarcoma. Med Arh 2012;66(1):68-69.
- 15. Chan V, Neumann D. Small cell lung carcinoma invading the pulmonary vein and left atrium as imaged by PET/CT. Eur J Nucl Med Mol Imaging 2005 Dec;32(12):1493.
- 16. Malm B, Hull S, Jadbabaie F. Left upper pulmonary vein thrombus in a patient with atrial fibrillation and prior lobectomy. Am J Med 2014 Nov;127(11):e7-e8.
- 17. Ohtaka K, Hida Y, Kaga K, Takahashi Y, Kawase H, Hayama S, et al. Left upper lobectomy can be a risk factor for thrombosis in the pulmonary vein stump. J Cardiothorac Surg 2014 Jan;9:5.
- 18. Yataco J, Stoller JK. Pulmonary venous thrombosis and infarction complicating pulmonary venous stenosis following radiofrequency ablation. Respir Care 2004 Dec;49(12):1525-1527.
- 19. López-Reyes R, García-Ortega A, Torrents A, Feced L, Calvillo P, Libreros-Niño EA, et al. Pulmonary venous thrombosis secondary to radiofrequency ablation of the pulmonary veins. Respir Med Case Rep 2017 Dec;23:46-48.
- 20. Uhlmann EJ, Dunitz JM, Fiol ME. Pulmonary vein thrombosis after lung transplantation presenting as stroke. J Heart Lung Transplant 2009 Feb;28(2):209-210.
- 21. Ohtaka K, Hida Y, Kaga K, Iimura Y, Shiina N, Muto J, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2012 Jan;143(1):e3-e5.
- 22. Nagaoka E, Yano M, Sugano T, Miyamoto T. Thrombus in the left superior pulmonary vein after left upper pulmonary lobectomy. J Thorac Cardiovasc Surg 2008 Mar;135(3):709-710.
- 23. Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr 2004 May;17(5):487-488.
- 24. Seki M, Endo M, Kidani M, Kobayashi H, Sato H, Noto T. [A rare case of left atrial thrombus after left upper pulmonary lobectomy]. Nihon Kyobu Geka Gakkai Zasshi 1989 Jul;37(7):1371-1375.
- 25. Asai K, Mochizuki T, Iizuka S, Momiki S, Suzuki K. Pulmonary vein stump thrombus: an early complication following upper division segmentectomy of the left lung. Gen Thorac Cardiovasc Surg 2014 Apr;62(4):244-247.
- 26. Girod JP, Lopez-Candales A. Pulmonary vein thrombosis in the setting of blunt chest trauma. J Am Soc Echocardiogr 2007 Dec;20(12):1416.e1.
- 27. Selvidge SD, Gavant ML. Idiopathic pulmonary vein thrombosis: detection by CT and MR imaging. AJR Am J Roentgenol 1999 Jun;172(6):1639-1641.
- 28. Lijfering WM, Coppens M, Veeger NJ, Middeldorp S, Hamulyák K, Prins MH, et al. Hyperhomocysteinemia is not a risk factor for venous and arterial thrombosis, and is associated with elevated factor VIII levels. Thromb Res 2008;123(2):244-250.
- 29. Shah AS, Michler RE, Downey RJ, Leibowitz DW, Homma S, Smith CR. Management strategies for pulmonary vein thrombosis following single lung transplantation. J Card Surg 1995 Mar;10(2):169-178.
- 30. Huang YC, Cheng YJ, Lin YH, Wang MJ, Tsai SK. Graft failure caused by pulmonary venous obstruction diagnosed by intraoperative transesophageal echocardiography during lung transplantation. Anesth Analg 2000 Sep;91(3):558-560.
- 31. Nahar T, Savoia MT, Liguori C, DiTullio MR, Schulman LL, Homma S. Spontaneous resolution of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr 1998 Feb;11(2):209-212.
- 32. Bonnet L, Raposo N, Blot-Souletie N, Faruch Bilfeld M, Chollet F, Mazière J, et al. Stroke caused by a pulmonary vein thrombosis revealing a metastatic choriocarcinoma. Circulation 2015 Jun;131(23):2093-2094.
- 33. Grau AJ, Schoenberg SO, Lichy C, Buggle F, Bock M, Hacke W. Lack of evidence for pulmonary venous thrombosis in cryptogenic stroke: a magnetic resonance angiography study. Stroke 2002 May;33(5):1416-1419.
- 34. Manabe S, Oshima Y, Nakano M, Fujii T, Maehara T, Nitta K, et al. Renal infarction in a patient with pulmonary vein thrombosis after left upper lobectomy. Case Rep Nephrol Urol 2014 May;4(2):103-108.
- 35. Schulman LL, Anandarangam T, Leibowitz DW, Ditullio MR, McGregor CC, Galantowicz ME, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr 2001 Aug;14(8):806-812.
- 36. Chaaya G, Vishnubhotla P. Pulmonary vein thrombosis: a recent systematic review. Cureus 2017 Jan;9(1):e993.
- 37. Alexander GR, Reddi A, Reddy D. Idiopathic pulmonary vein thrombosis: a rare cause of massive hemoptysis. Ann Thorac Surg 2009 Jul;88(1):281-283.
- 38. Vazquez FJ, Paulin P, Rodriguez P, Lubertino M, Gándara E. The outcome of pulmonary vein thrombosis in non-surgical patients. A systematic review and case report. Thromb Haemost 2015 May;113(5):1151-1154.
- 39. Alikhan R, Gomez K, Maraveyas A, Noble S, Young A, Thomas M. Cancer-associated venous thrombosis in adults: a British society for haematology guideline. 2nd ed. 2024. p. 1-17.
- 40. Streiff MB, Abutalib SA, Farge D, Murphy M, Connors JM, Piazza G. Update on guidelines for the management of cancer-associated thrombosis. Oncologist 2021 Jan;26(1):e24-e40.
- 41. Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, et al. American society of hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv 2021 Feb;5(4):927-974.
- 42. Amemiya T, Shono T, Yamagami K, Takagishi S, Toma H, Kobarai T, et al. Usefulness of oral Xa inhibitor for management of ischemic stroke associated with thrombosis in the pulmonary vein stump after lung resection. J Stroke Cerebrovasc Dis 2019 Nov;28(11):104321.
- 43. Lebel K, Tan S, Parent MC, El-Hamamsy I, Couture C, Laroussi L. An unusual presentation of a cardiac thrombus. Can J Cardiol 2019 Oct;35(10):1420.e1-1420.e3.
- 44. Takeuchi H. The desirable effects of edoxaban on thrombi in the left atrium are seemingly connected to pulmonary vein thrombi. Cureus 2024 Jan;16(1):e52337.
- 45. Ngo D, Aftab G, Madhavan A, Bukhari A. Idiopathic pulmonary vein thrombosis treated with apixaban. Respirol Case Rep 2021 Jul;9(8):e00803.
- 46. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014 Mar;383(9921):955-962.
- 47. van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014 Sep;124(12):1968-1975.
- 48. Frere C, Farge D, Schrag D, Prata PH, Connors JM. Direct oral anticoagulant versus low molecular weight heparin for the treatment of cancer-associated venous thromboembolism: 2022 updated systematic review and meta-analysis of randomized controlled trials. J Hematol Oncol 2022 May;15(1):69.
- 49. Alsubaie NS, Al Rammah SM, Alshouimi RA, Alzahrani MY, Al Yami MS, Almutairi AR, et al. The use of direct oral anticoagulants for thromboprophylaxis or treatment of cancer-associated venous thromboembolism: a meta-analysis and review of the guidelines. Thromb J 2021 Oct;19(1):76.
- 50. Schmid C, Gulba DC, Heublein B, Kemnitz J, Haverich A. Systemic recombinant tissue plasminogen activator lysis for left atrial thrombus formation after single-lung retransplantation. Ann Thorac Surg 1992 Feb;53(2):338-340.