Heterotopic pancreas (HP) is a pancreatic tissue found at a location distant from where it is usually located. Although not anatomically related to the normal pancreas, the ectopic tissue can develop different pathological pancreatic lesions.1 Here, we report a case of pancreatic intraepithelial neoplasia (PanIN) incidentally found in a duodenal HP with a synchronous invasive ductal carcinoma involving the pancreas.
Case Report
A 77-year-old man presented with an intermittent fever, widespread pruritus, and jaundice. Computed tomography, ultrasound, and magnetic resonance cholangiopancreatography revealed a pancreatic head mass with characteristics favoring adenocarcinoma. None of the imaging modalities indicated the presence of a submucosal lesion involving the duodenum.
The patient underwent a Whipple procedure. A firm, ill-defined mass surrounding the intrapancreatic common bile duct and pancreas head was detected. The duodenum had a well-defined intramural lesion with a consistently yellow sliced surface measuring 2 cm in maximum dimension. The overlying mucosa was unremarkable. Figure 1 shows the excised mass.
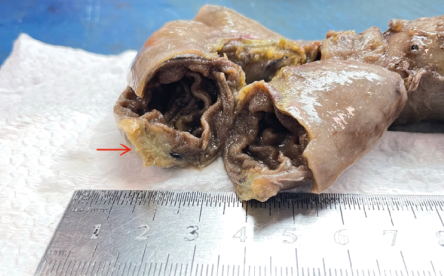 Figure 1: A gross image of the excised submucosal ectopic pancreatic foci. The red arrow indicates where it was attached to the duodenal wall. The cut surface displays a well-defined homogenous solid lesion.
Figure 1: A gross image of the excised submucosal ectopic pancreatic foci. The red arrow indicates where it was attached to the duodenal wall. The cut surface displays a well-defined homogenous solid lesion.
Microscopy of the pancreatic head mass revealed a moderately differentiated pancreatic ductal adenocarcinoma (PDAC). The surrounding pancreatic parenchyma showed a high-grade PanIN (HG-PanIN). Microscopic examination of the duodenal lesion revealed ectopic pancreatic tissue with low-grade PanIN (LG-PanIN) [Figure 2].
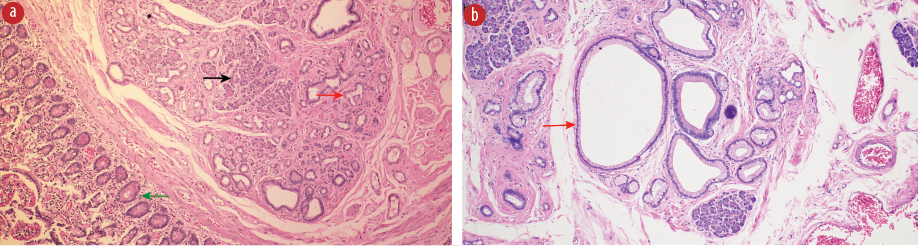 Figure 3: (a) The pancreatic ductal adenocarcinoma in the native pancreas expressing p53 immunohistochemistry stain with variable nuclear expression (stained brown), hematoxylin and eosin (H&E) stain, magnification = 10 ×. (b) The pancreatic intraepithelial neoplasia involving the ectopic pancreas shows complete loss of p53 nuclear stain (red arrow), H&E stain, magnification = 10 ×.
Figure 3: (a) The pancreatic ductal adenocarcinoma in the native pancreas expressing p53 immunohistochemistry stain with variable nuclear expression (stained brown), hematoxylin and eosin (H&E) stain, magnification = 10 ×. (b) The pancreatic intraepithelial neoplasia involving the ectopic pancreas shows complete loss of p53 nuclear stain (red arrow), H&E stain, magnification = 10 ×.
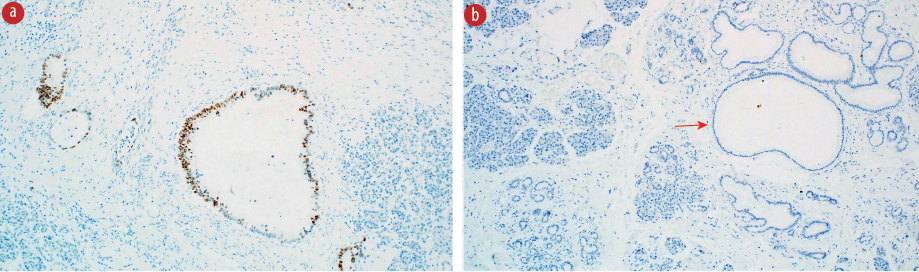 Figure 2: (a) A microscopic image of the ectopic submucosal pancreatic tissue composed of acini and ducts (black arrow) with a low-grade pancreatic intraepithelial neoplasia (LG-PanIN) lesion (red arrow) and overlying unremarkable duodenal mucosa (green arrow), hematoxylin and eosin (H&E) stain, magnification = 4 ×. (b) A higher magnification of the ectopic foci exhibiting LG-PanIN lesion (red arrow), H&E stain, magnification = 10 ×.
Figure 2: (a) A microscopic image of the ectopic submucosal pancreatic tissue composed of acini and ducts (black arrow) with a low-grade pancreatic intraepithelial neoplasia (LG-PanIN) lesion (red arrow) and overlying unremarkable duodenal mucosa (green arrow), hematoxylin and eosin (H&E) stain, magnification = 4 ×. (b) A higher magnification of the ectopic foci exhibiting LG-PanIN lesion (red arrow), H&E stain, magnification = 10 ×.
Immunohistochemical analysis using p16 and p53 markers showed a complete loss of p16 in both PDAC and ectopic LG-PanIN. The p53 revealed variable expression (i.e., wild-type staining pattern) in PDAC and no expression in ectopic PanIN (i.e., aberrant staining pattern) [Figure 3].
Shortly after the above procedure, the patient passed away due to complications related to his primary disease.
Discussion
HP tissues are generally found in unusual locations, each complete with its own vascular supply and duct system.1,2 They typically appear in the upper gastrointestinal tract (GIT), especially the duodenum.2,3 Several hypotheses have been proposed to explain this phenomenon, including totipotent endodermal cell development into mature pancreatic tissue, misplacement of pancreatic tissue during GIT formation, and metaplastic pancreatic tissue migration from the GIT mucosal surface to the submucosa.4–6
HP affects 0.5–13.7% of the population, and is three times more common in males than in females.5 As most HP lack symptoms, they are typically discovered incidentally during endoscopic or imaging workup, postoperatively, or at autopsy.1,2,6–9 The most commonly reported symptoms are vomiting, nausea, and weight loss.2,5 A series of studies discovered that symptomatic HP lesions are likely to be > 2 cm in size, occur more frequently in younger individuals, are associated with a lymphoid cuff, and are more likely to be located in the stomach.10 Larger lesions are related to more severe symptoms.8 Moreover, HP lesions are associated with acute pancreatitis, neoplasia, and other pathologies that can occur in the normal pancreas. Malignant conditions, such as in our patient, are extremely rare.1,11
Diagnosing HP based on imaging is challenging.6,11 On endoscopic ultrasound, an HP lesion may appear as a poorly defined, homogeneous, hypoechoic lesion that may be mistaken for acinous components or dispersed adipose tissue.6 Leiomyoma, gastrointestinal stromal tumor, and other lesions with comparable characteristics might also be included in the differential diagnosis.12 Endoscopic ultrasound-guided fine needle aspiration might be the most reliable modality to confirm the diagnosis.1
The HP in the GIT presents macroscopically as a hard, well-defined subepithelial lesion that can be comparable to a gastrointestinal stromal tumor or leiomyoma.12 Microscopically, it comprises a variable combination of normal pancreatic constituents, including acini, ducts, or islet cells.5 Type I is distinguished by the presence of all three elements; type II by the predominance of acini without islet cells; and type III by the presence of pancreatic ducts without islet cells.6,12 They are typically seen in the submucosa, but may also be found elsewhere. Submucosal lesions can be missed during endoscopic biopsies due to the limited depth of the procedure, so using endoscopic ultrasound-guided fine needle aspiration has been shown to provide superior results.1,12
A Whipple procedure enabled us to discover an incidental PanIN lesion in an ectopic pancreas. PanIN is usually described as a microscopic (< 5 mm) non-invasive neoplastic lesion arising within the pancreatic ducts.13 It is the most important risk factor for pancreatic invasive ductal adenocarcinoma.4 PanIN lesions are classified as low- and high-grade lesions, depending on their degree of cytological and architectural atypia.13,14
The PanIN incidence increases with age. LG-PanINs are more frequent and can be found in over 50% of people over the age of 50.15 They either progress to a HG-PanIN and eventually develop into a carcinoma—as in our patient—or persist as low-grade for decades.16,17 On the other hand, the risk of developing cancer from an HG-PanIN is reported to be as high as 33%.16 LG-PanIN can seldom directly progress to cancer, and is reported only in 1.5% of males and 1.3% of females with the condition.
In the present case, microscopy found moderately differentiated PDAC. Molecular studies support the hypothesis that PanIN can progress to PDAC with the accumulation of genetic alterations over time.4,16,17 The majority of LG-PanINs harbor telomerase shortening and Kirsten rat sarcoma viral oncogene homologue activating point mutation, both of which are thought to represent early molecular aberrations that lead to PDCA.14,18
At the intermediate stages of neoplasm growth, the tumor suppressor gene, cyclin-dependent kinase inhibitor 2A/p16, is inactivated, which favors the establishment of an HG-PanIN.14,15,17,18 This is present in the great majority of pancreatic carcinomas.4 In the late stages (i.e., invasive PDAC), the tumor suppressor genes TP53 and SMAD4 are frequently inactivated.14,18 The TP53 gene encodes for the p53 protein, which regulates cell division and apoptosis. The absence of this protein facilitates the accumulation of genetic variations, which promotes cell growth and survival.4,19 The SMAD4 protein is also important in cell cycle control.4
One study found that HG-PanIN-associated PDAC carried more Kirsten rat sarcoma viral oncogene homologue mutations, while PDAC with no HG-PanIN in the background harbored TP53 and SMAD4 inactivating mutations.14 This highlights the possible different molecular pathways from which PDAC can arise, including the current case where the p53 exhibited wild-type expression in the PDAC but aberrant expression in the ectopic LG-PanIN.
Our patient had PanIN lesions arising within his HP lesion. Due to this phenomenon rarity, treatment and follow-up guidelines have not been established. Endoscopic resection has been suggested with endoscopic mucosal resection or endoscopic submucosal dissection if endoscopic mucosal resection is difficult.1,6 HP PanIN lesions are best treated surgically due to the risk of bleeding and perforation during endoscopic excision. Safe resection margins are undefined.1,5 It’s unclear whether these patients require further follow-up.
Conclusion
A PanIN arising within an HP is rare. However, it is important to identify it due to the risk of progression to an invasive carcinoma. More data is required to establish guidelines for the appropriate management and follow-up of HP PanIN.
Disclosure
The authors declare no conflicts of interest. Since the patient had passed away, ethical approval was obtained from the hospital administration for publication of this case report.
references
- 1. Safadi S, Martin DR, Rustagi T. Pancreatic intraepithelial neoplasia in heterotopic pancreas: incidentally diagnosed on endoscopic mucosal resection of a duodenal polyp. BMJ Case Rep 2018 Jun;2018:bcr2018224414.
- 2. Persano G, Cantone N, Pani E, Ciardini E, Noccioli B. Heterotopic pancreas in the gastrointestinal tract in children: a single-center experience and a review of the literature. Ital J Pediatr 2019 Nov;45(1):142.
- 3. Sathyanarayana SA, Deutsch GB, Bajaj J, Friedman B, Bansal R, Molmenti E, et al. Ectopic pancreas: a diagnostic dilemma. Int J Angiol 2012 Sep;21(3):177-180.
- 4. Distler M, Aust D, Weitz J, Pilarsky C, Grützmann R. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. Biomed Res Int 2014;2014:474905.
- 5. Farah A, Mansour S, Khuri S. Gastrointestinal tract heterotopic pancreas: asymptomatic pathology? Gastroenterology Res 2021 Feb;14(1):45-47.
- 6. Yang YB, Liu YQ, Dai L, Yan WP, Liang Z, Chen KN. Malignant transformation of heterotopic pancreas as middle esophagus adenocarcinoma-a rare case report and comprehensive literature review. Thorac Cancer 2022 Apr;13(7):1083-1087.
- 7. Jansen van Rensburg A, Raufian K. A case report of ectopic pancreatic tissue in the gallbladder. J Surg Case Rep 2022 Mar;2022(3):rjac098.
- 8. Paramythiotis D, Kollatou AS, Simou T, Karlafti E, Abba Deka I, Petrakis G, et al. Ectopic pancreatic tissue in stomach: a case report. Ann Med Surg (Lond) 2022 Jul;79:104005.
- 9. Mickuniene R, Stundiene I, Jucaitis T, Valanciene D, Valantinas J. A case of ectopic pancreas in the ileum presenting as obscure gastrointestinal bleeding and abdominal pain. BMC Gastroenterol 2019 Apr;19(1):57.
- 10. Jun SY, Son D, Kim MJ, Kim SJ, An S, Park YS, et al. Heterotopic pancreas of the gastrointestinal tract and associated precursor and cancerous lesions: systematic pathologic studies of 165 cases. Am J Surg Pathol 2017 Jun;41(6):833-848.
- 11. Rosales Torres P, Pila Pérez R, León Acosta P, Pila Peláez R. A case of heterotopic pancreas in a gastric polyp. Rev Colomb Gastroenterol 2019;34(3):293-296.
- 12. Betzler A, Mees ST, Pump J, Schölch S, Zimmermann C, Aust DE, et al. Clinical impact of duodenal pancreatic heterotopia – is there a need for surgical treatment? BMC Surg 2017;17(1):53.
- 13. Morani AC, Hanafy AK, Ramani NS, Katabathina VS, Yedururi S, Dasyam AK, et al. Hereditary and sporadic pancreatic ductal adenocarcinoma: current update on genetics. Radiol Imaging Cancer 2020 Mar;2(2):e190020.
- 14. Basturk O, Esposito I, Klöppel G, Furukawa T, Zamboni G, Fukushima N, et al. Tumors of the pancreas: benign epithelial tumors and precursors. In: WHO classification of tumors. 5th ed. International Agency on Research on Cancer (IARC); 2019. p. 307-309.
- 15. Miyazaki T, Ohishi Y, Miyasaka Y, Oda Y, Aishima S, Ozono K, et al. Molecular characteristics of pancreatic ductal adenocarcinomas with high-grade pancreatic intraepithelial neoplasia (Panin) are different from those without high-grade PanIN. Pathobiology 2017;84(4):192-201.
- 16. Peters ML, Eckel A, Mueller PP, Tramontano AC, Weaver DT, Lietz A, et al. Progression to pancreatic ductal adenocarcinoma from pancreatic intraepithelial neoplasia: results of a simulation model. Pancreatology 2018 Dec;18(8):928-934.
- 17. Hong SM, Heaphy CM, Shi C, Eo SH, Cho H, Meeker AK, et al. Telomeres are shortened in acinar-to-ductal metaplasia lesions associated with pancreatic intraepithelial neoplasia but not in isolated acinar-to-ductal metaplasias. Mod Pathol 2011 Feb;24(2):256-266.
- 18. Hosoda W, Chianchiano P, Griffin JF, Pittman ME, Brosens LA, Noë M, et al. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J Pathol 2017 May;242(1):16-23.
- 19. Aubrey BJ, Strasser A, Kelly GL. Tumor-suppressor functions of the TP53 pathway. Cold Spring Harb Perspect Med 2016 May;6(5):a026062.