|
Abstract
Cardiogenic embolism is a major cause of stroke and often leads to significant morbidity and mortality. Despite the recent advances in our understanding of the pathophysiology of stroke and its risk factors, diagnosis and therapy; some case scenarios still present a real challenge for the treating physicians. We report a case of a 50 year old male patient presenting with multi-territory cerebral infarctions due to a left ventricular mobile thrombus complicated with hemorrhagic transformation at the time of presentation. Gradual introduction of anticoagulation coupled with a multidisciplinary team approach advocating careful daily clinical assessment of the patient and regular echocardiographic and neuroimaging studies have resulted in a better management and achievement of therapeutic goals.
Keywords: Stroke; Cardioembolism; Mobile thrombus; Hemorrhagic transformation; Echocardiography; Multidisciplinary approach.
Introduction
Embolic stroke is a leading cause of morbidity and mortality. The heart is an important source for the development of embolic strokes. About 15-20% of all ischemic strokes are cardioembolic. Establishing the heart as a source for a patient's embolic stroke carries important prognostic and therapeutic implications. Patients with such types of stroke often suffer significant morbidity and mortality and have a high risk of recurrence. The presence of a left ventricular thrombus carries additional risks including the risk of hemorrhagic transformation as well as a high risk for recurrent embolic strokes. Managing patients with left ventricular thrombi causing an embolic stroke is challenging especially in the presence of hemorrhagic transformation as we present in this report.
Case Report
A 50-year old right-handed Omani male with a history of smoking and dyslipidemia, taking aspirin and simvastatin, presented with 6 hours history of sudden onset left sided weakness and inability to speak. He had no history of chest pain or palpitations. He did, however, give a history of a recent admission to another hospital with an episode of prolonged chest pain. Details of his management during that admission were not available. Examination showed blood pressure of 115/80 mmHg, regular heart rate of 90 per minute, Glasgow coma scale (GCS) of 12, expressive aphasia, left facial nerve palsy of upper motor neuron type with dense left sided hemiplegia and left homonymous hemianopsia (NIH Stroke Scale score 17). Cardiac examination as well as ECG, cardiac enzymes and basic laboratory investigations was unremarkable. Initial CT brain showed a right occipital infarct with focal areas of hemorrhagic transformation. The patient was admitted to the high dependency unit of the general medical ward with regular assessment of his GCS. A repeat CT brain was performed on the second day and showed a large right middle cerebral artery (MCA) territory infarct with mass effect in addition to the right occipital infarction and the hemorrhagic transformation noted earlier. (Fig. 1)
Echocardiography revealed poor overall systolic function (ejection fraction 45%) with hypokinetic apical and lateral walls with an apical aneurysm and a mobile thrombus measuring about 3.6 cm × 1.7 cm in the apical region of the left ventricle (Fig. 2). The patient was started on 100 ml of Mannitol 20% three times daily with daily monitoring of the electrolytes profile in addition to regular physiotherapy. Forty-eight hours after the onset, he was started on weight-adjusted low molecular weight heparin (LMWH) at a prophylactic dose (enoxaparin 40 mg/day) and clopidogrel 75 mg once a day. He had minimal improvement in his neurological status. His thrombophilia workup (including tests for protein S, protein C and anti-thrombin levels and activities sent before the initiation of LMWH, as well as molecular testing for factor V Leiden and prothrombin gene mutations) were unremarkable. MRI brain was done and confirmed the CT findings.
Daily assessment of the patient by a multi-disciplinary team including a neurologist, a cardiologist and a hematologist in addition to the primary internist was adopted. A weekly echocardiogram was used to assess the thrombus size while weekly neuro-imaging with CT scans was initially used to assess the infracted areas, especially with regards to hemorrhagic transformation. With no progression in the small areas of hemorrhagic transformation on CT scans, the dose of enoxaparin was upgraded to 40 mg twice daily and then after another week, it was increased to 60 mg twice daily with weekly monitoring of anti-Xa levels. Warfarin was introduced with a target INR of 2.0 to 3.0 when the CT showed complete resolution of the cerebral hemorrhage. Follow up echocardiographic studies demonstrated a significant regression in the thrombus size without any evidence of further systemic embolization. The patient was discharged after 7 weeks of hospitalization with NIH Stroke Scale score of 15 and a modified Rankin Scale score of 5.
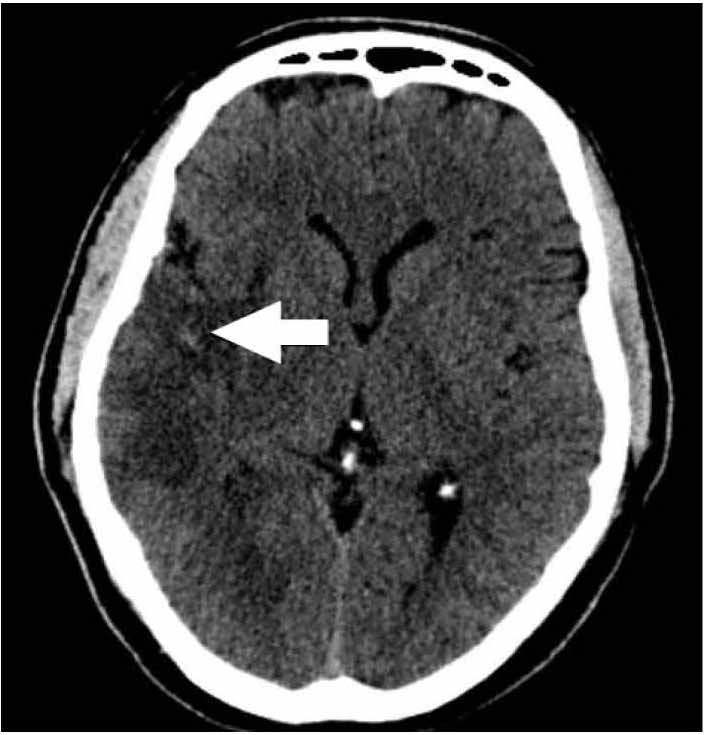
Figure 1: The patient's initial CT brain showing multiple areas of ischemic infarcts, the largest of which is in the distribution of the right middle cerebral artery, with a small area of focal hemorrhagic transformation (arrow).
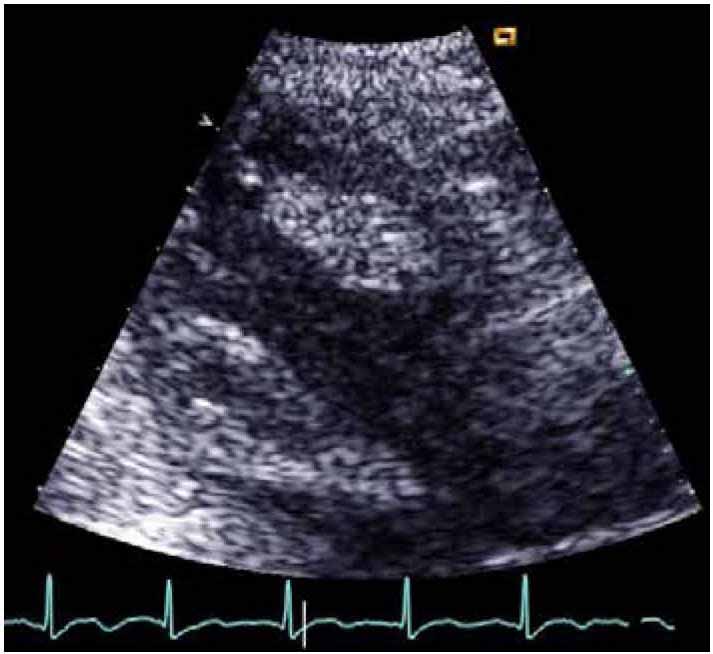
Figure 2: Echo showing the LV thrombus.
Discussion
Cardiogenic embolism is recognized to account for one in six strokes.1,2 Common causes of cardiac embolization include atrial fibrillation, ischemic heart disease, rheumatic heart disease and prosthetic cardiac valves.3-6 Left ventricular thrombi (a frequent complication of transmural acute myocardial infarction, likely to be the case in our patient),7,8 are second to atrial thrombi from atrial fibrillation as a cause of cardiogenic embolization. These are easily detectable by two-dimensional echocardiography, although false-positive studies exist.9 Protruding configuration and free mobility of the thrombus and the presence of adjacent hypokinesia indicate a high embolization potential.10,11 The presence of a potential embolic source does not, by itself, justify the diagnosis of cardiogenic embolism in this patient. However, the abrupt onset of maximal deficit, the presence of multiple brain infarcts involving different vascular territories with hemorrhagic transformation along with the presence of a large left ventricular thrombus indicate that the cause of the infarcts in this patient are indeed cardioembolic.
Hemorrhagic transformation (HT) is not uncommon and patients with cardioembolic infarcts are particularly prone to develop hemorrhagic transformation. HT has been reported in up to 40% of patients with cerebral embolism on CT scans,12 and more than 60% on MRI.13 Older age and large sized infarcts are known to be associated with particularly high risks of hemorrhagic transformation.12-14 In spite of his young age, our patient suffered large cardioembolic infarcts in multiple vascular territories, which led to hemorrhagic transformation.
Patients like the one we are reporting pose a significant challenge. On one hand, he had a major source for recurrent embolic stroke. On the other hand, treating that source with systemic anticoagulation carries the risk of exacerbating the pre-existing cerebral hemorrhage, especially given the size of his initial infarcts. The decision to anticoagulate is generally based on the balance between the relative risk of these two, i.e. the relative risk of embolization without anticoagulation and that of rebleeding or expansion of existing bleeds if anticoagulants are started early. Although the exact risk of each of these is difficult to define, our patient had high risks for both. An extensive literature search to guide our strategy in managing this patient was largely unhelpful and revealed contradicting data. Similar cases are rarely reported in the literature.15 Unfortunately, the available guidelines for the management of stroke do not address this particular issue.16-18 Properly designed clinical trials of the optimal anticoagulation strategy in cardiogenic embolism are not available.19 Several studies have reported no increase in the rate of hemorrhagic transformation, hence supporting the use of immediate anticoagulation in embolic stroke.20-24 However, such studies are either retrospective, including small numbers of patients or, more importantly, have excluded patients with hemorrhagic transformation on initial presentation. Several other studies, on the hand, have shown either an increase in intra-cranial bleeding or no significant reduction in the recurrence of ischemic strokes, death or disability.25-28
As our patient had spontaneous hemorrhagic transformation in a large embolic infarct (both of which are associated with increased risk of further hemorrhage), we opted not to initiate full anticoagulation. This approach is supported by evidence from several reports on patients with high risk of embolization and urgent need for anticoagulation (such as patients with mechanical heart valves) who present with intra-cranial hemorrhage.29-33 Such reports indicate that it is safe to withhold anticoagulation for periods extending up to a few weeks. The challenge, however, was that with his large mobile left ventricular thrombus, he had another large embolic infarct within a day of the initial one and without anticoagulation; one risks him getting another major one. We therefore, opted for a graded introduction of anticoagulation initially with prophylactic doses of LMWH going to full dose with close monitoring of anti-Xa levels, as well as regular imaging with CT scans and echocardiography. The patient did not suffer any further emboli or more hemorrhage. He in fact, showed some improvement both clinically and in the size of the left ventricular thrombus.
Conclusion
Left ventricular thrombus complicating ischemic heart disease and causing systemic embolism is a well-recognized clinical phenomenon which requires anticoagulation. This therapy may pose a real challenge in patients with ischemic strokes complicated by hemorrhagic transformation. Multidisciplinary approaches, gradual introduction of anticoagulation guided by the available literature and close monitoring in such patients would result in a better outcome.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Mohr JP, Caplan LR, Melski JW, Goldstein RJ, Duncan GW, Kistler JP, et al. The Harvard Cooperative Stroke Registry: a prospective registry. Neurology 1978 Aug;28(8):754-762.
2. Robertson JT. Neck manipulation as a cause of stroke. Stroke 1981 Jan-Feb;12(1):1.
3. Cerebral Embolism Task Force. Cardiogenic brain embolism. Arch Neurol 1986 Jan;43(1):71-84.
4. Cerebral Embolism Task Force. Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol 1989 Jul;46(7):727-743.
5. Stratton JR. Common causes of cardiac emboli–left ventricular thrombi and atrial fibrillation. West J Med 1989 Aug;151(2):172-179.
6. Pujadas Capmany R, Arboix A, Casañas-Muñoz R, Anguera-Ferrando N. Specific cardiac disorders in 402 consecutive patients with ischaemic cardioembolic stroke. Int J Cardiol 2004 Jun;95(2-3):129-134.
7. Stokman PJ, Nandra CS, Asinger RW. Left Ventricular Thrombus. Curr Treat Options Cardiovasc Med 2001 Dec;3(6):515-521.
8. Easton JD, Sherman DG. Management of cerebral embolism of cardiac origin. Stroke 1980 Sep-Oct;11(5):433-442.
9. Stratton JR, Lighty GW Jr, Pearlman AS, Ritchie JL. Detection of left ventricular thrombus by two-dimensional echocardiography: sensitivity, specificity, and causes of uncertainty. Circulation 1982 Jul;66(1):156-166.
10. Visser CA, Kan G, Meltzer RS, Dunning AJ, Roelandt J. Embolic potential of left ventricular thrombus after myocardial infarction: a two-dimensional echocardiographic study of 119 patients. J Am Coll Cardiol 1985 Jun;5(6):1276-1280.
11. Jugdutt BI, Sivaram CA. Prospective two-dimensional echocardiographic evaluation of left ventricular thrombus and embolism after acute myocardial infarction. J Am Coll Cardiol 1989 Mar;13(3):554-564.
12. Okada Y, Yamaguchi T, Minematsu K, Miyashita T, Sawada T, Sadoshima S, et al. Hemorrhagic transformation in cerebral embolism. Stroke 1989 May;20(5):598-603.
13. Hornig CR, Bauer T, Simon C, Trittmacher S, Dorndorf W. Hemorrhagic transformation in cardioembolic cerebral infarction. Stroke 1993 Mar;24(3):465-468.
14. Hornig CR, Dorndorf W, Agnoli AL. Hemorrhagic cerebral infarction–a prospective study. Stroke 1986 Mar-Apr;17(2):179-185.
15. Misumi I, Kimura Y, Hokamura Y, Honda Y, Misumi K, Yamabe H, et al. Acute left ventricular dysfunction and left ventricular thrombus in a patient with cerebral hemorrhage. Intern Med 1997 Feb;36(2):92-96.
16. Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008 Jun;133(6)(Suppl):630S-669S.
17. Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2011 Jan;42(1):227-276.
18. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al; Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists Circulation 2007 May;115(20):e478-e534.
19. Fuller R, Dudley NJ, Maule J, Stewart T. Dilemmas in managing intracerebral haemorrhage and thromboembolism. J R Soc Med 2004 Jun;97(6):308-309.
20. Bertram M, Bonsanto M, Hacke W, Schwab S. Managing the therapeutic dilemma: patients with spontaneous intracerebral hemorrhage and urgent need for anticoagulation. J Neurol 2000 Mar;247(3):209-214.
21. Cerebral Embolism Study Group. Immediate anticoagulation of embolic stroke: a randomized trial. Stroke 1983 Sep-Oct;14(5):668-676.
22. ESPRIT. Oral anticoagulation in patients after cerebral ischemia of arterial origin and risk of intracranial hemorrhage. Stroke 2003 Jun;34(6):e45-e46. Published online 1 May 2003.
23. Chamorro A, Vila N, Saiz A, Alday M, Tolosa E. Early anticoagulation after large cerebral embolic infarction: a safety study. Neurology 1995 May;45(5):861-865.
24. Lodder J, van der Lugt PJ. Evaluation of the risk of immediate anticoagulant treatment in patients with embolic stroke of cardiac origin. Stroke 1983 Jan-Feb;14(1):42-46.
25. Cerebral Embolism Study Group. Immediate anticoagulation of embolic stroke: brain hemorrhage and management options. Stroke 1984 Sep-Oct;15(5):779-789.
26. Moonis M, Fisher M. Considering the role of heparin and low-molecular-weight heparins in acute ischemic stroke. Stroke 2002 Jul;33(7):1927-1933.
27. Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: a meta-analysis of randomized controlled trials. Stroke 2007 Feb;38(2):423-430. Published online 4 Jan 2007.
28. Bath PM, Iddenden R, Bath FJ. Low-molecular-weight heparins and heparinoids in acute ischemic stroke : a meta-analysis of randomized controlled trials. Stroke 2000 Jul;31(7):1770-1778.
29. Phan TG, Koh M, Wijdicks EF. Safety of discontinuation of anticoagulation in patients with intracranial hemorrhage at high thromboembolic risk. Arch Neurol 2000 Dec;57(12):1710-1713.
30. Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest 2001 Feb;119(2):478-484.
31. Butler A, Tait RC. Restarting oral anticoagulation after intracranial hemorrhage. Stroke 2004 Jan; 35(1):e5-6; author reply e5-6.
32. Babikian VL, Kase CS, Pessin MS, Caplan LR, Gorelick PB. Resumption of anticoagulation after intracranial bleeding in patients with prosthetic heart valves. Stroke 1988 Mar;19(3):407-408.
33. Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010 Dec;41(12):2860-2866. Published online 28 Oct 2010.
|