Staphylococcus aureus (S. aureus) is an important pathogen responsible for a wide range of human infections, including minor skin infections, pimples, impetigo, boils, cellulitis, folliculitis, carbuncles, scalded skin syndrome, and abscesses, including life-threatening diseases.1,2 S. aureus is an important pathogen of many nosocomial and community-related infections leading to high morbidity and mortality.3 S. aureus possesses various antibiotic resistance mechanisms, including resistance to methicillin known as methicillin-resistant S. aureus (MRSA), which consequently becomes difficult in managing infections. Over the last 50 years, antibiotics have reduced the rate of mortality; nevertheless, bacteria have been known to develop maximum resistance to most of the available antimicrobial agents.4
The methicillin resistance expressed by S. aureus is contributed by the mecA gene that is harbored by the mobile segments of the MRSA strains, which encodes the penicillin-binding protein 2a that has a low affinity for β-lactam and allows MRSA strains to survive in different concentrations of these antimicrobial agents.5 It is known that MRSA is endemic in India with variation in the antimicrobial susceptibility patterns based on geographical region.6 Early detection of MRSA and its susceptibility pattern becomes vital for the treatment of the condition as very few antimicrobial agents can be used to manage the ailment. Hence, it is imperative to study the overall prevalence of MRSA in India to develop improved and efficient treatment methods for its management.
Our study concentrates on systematic review and meta-analysis to estimate the pooled prevalence of MRSA in India and state-wise, zone-wise, and year-wise analysis was conducted using statistical tools, viz., meta-analysis.
Methods
Literature search
We performed a systematic search for articles using the following keywords in various combinations: ‘Staphylococcus aureus’, ‘S. aureus’, ‘MRSA’, ‘prevalence’, ‘India’, and ‘Humans’. We used various search engines such as J-Gate Plus, PubMed, Google Scholar, and Indian journals. The search was limited to articles published from 2015 to 2020. In addition, manual searches on citations retrieved from original studies and review articles were also performed. Finally, the articles were chosen by screening through the titles and abstracts for relevance based on the inclusion and exclusion criteria.
Study selection criteria
The results after searching were tabulated into Excel, duplicates were removed, and relevant studies were examined. Our preliminary inclusion criteria were to include all articles having the title keyword “prevalence of MRSA in India” from 2015 to 2020 only. Selected papers were subjected to abstract screening for titles. Studies were read in full for which they had reported on: (a) the prevalence of MRSA, (b) sample size data, (c) events (positive), (d) year of study, (e) geographical location of the study, and (f) diagnostic tests used as confirmatory tool for identification of MRSA. Those articles that did not satisfy the above screening criteria were excluded from the study. Articles containing a large number of samples/events were also not included in the study. Studies that did not report the MRSA prevalence included reviews, reports, editorial articles and outbreak reports, and studies that were duplicates of included studies were excluded. The articles that were selected included humans of all age groups. The searches, scrutiny, and methodology were in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol (http:// www.prisma-statement.org).
Data extraction
The data was extracted from qualified studies that included first author, year of publication, study setting/sampling location, number of investigated cases, number of MRSA isolates, sources of isolates, diagnostic methods employed for confirmation, antibiogram results, and considered for meta-analysis. We were also interested in the year of publication and the location of the study setting to stratify the studies based on the year of publication, zone-wise, and state-wise. Studies were independently extracted by two investigators and discussed to arrive at a consensus.
Risk of bias and quality assessment
The quality assessment of different studies was done on a fixed rating scale.7 The scoring was on a scale of 0 to 5, which included evaluation of author and year of study, representativeness of the sample used in the study, ascertainment of the exposure, comparability, and outcome.
Meta-analysis
Meta-analysis was performed using the R Open Source Scripting Software (version 3.4.3, R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/). Metafor, Metaprop, and Meta of this software were statistical packages used. Tau square, I2 (Higgins’ I2), and p-values were computed to determine the percentage of variation due to heterogeneity among various reports included in this study. The random-effect and fixed-effect models were used to calculate the pooled prevalence of individual diseases. This analysis facilitates generating a weighted average proportion of prevalence of various studies, providing a way forward for proper planning. Graphical representation of the data was depicted as forest plots. The restricted maximum-likelihood estimator was used to determine between-study variance (τ2). The prevalence estimates for MRSA were expressed as a percentage with 95% CI. Subgroup analysis was performed to investigate the significance of heterogeneity among the studies. The studies were stratified based on zones of the country, year of publication, and state-wise. Subgroup meta-regression analysis was performed to identify the stratified prevalence of MRSA in different regions, study periods, sample size, and diagnostic tests.

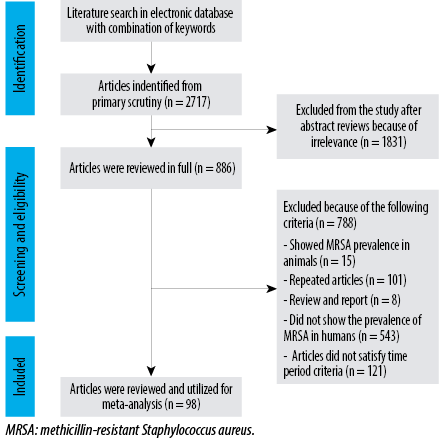 Figure 1: Systematic review and meta-analysis.
Figure 1: Systematic review and meta-analysis.
Results
Study details
Articles reporting the prevalence of MRSA were thoroughly screened, and irrelevant ones were excluded. A total of 1831 of 2717 articles identified were excluded following the exclusion criteria described above; 886 potential articles were selected using a combination of keywords. A total of 98 articles were selected suitable for systematic review and meta-analysis [Figure 1]. All the articles described the prevalence of MRSA in India and were published between 2015 and 2020. The prevalence data for this study were extracted and tabulated as per the requirement of the statistical software. Twenty-two states of India had reports of the prevalence of MRSA. Six zones of the country, namely; North (Uttar Pradesh, Haryana, Jammu and Kashmir, Himachal Pradesh, Punjab, New Delhi, and Uttarakhand), East (West Bengal and Odisha), West (Rajasthan, Maharashtra, and Gujarat), South (Tamil Nadu, Telangana, Karnataka, Andhra Pradesh, Kerala, and Puducherry), Central (Madhya Pradesh), and Northeast (Assam, Tripura, and Sikkim) zones had a varied pooled prevalence of MRSA.
Risk of bias and quality assessment
Risk of bias and quality assessment were awarded a maximum of two stars, and the score given was on a scale of 0 to 5. Hence, the overall quality assessment has a maximum score of 5 and a minimum score of 3.
Meta-analysis of the prevalence of MRSA
The percentage prevalence of MRSA in India was estimated statistically using R Open source Scripting software. The overall prevalence of MRSA using 17 525 samples in 98 studies was 37% (95% CI: 32–41) in India during 2015–2020 (I2 = -99%, τ2 = 0.0571, p < 0.001) [Table 1]. The pooled data were stratified into state-wise and zone-wise.
Twenty-two states of India have reported the prevalence of MRSA. Jammu and Kashmir showed the highest pooled prevalence of MRSA at 55% (95% CI: 42–67) with I2 = -88, τ2 = -0.0112, p < 0.01, and Maharashtra showed the lowest pooled prevalence of MRSA at 21% (95% CI: 11–34) with I2 = -99, τ2 = -0.0517, p < 0.01. A single article from Sikkim had a prevalence of MRSA as 35% (95% CI: 28–44) [Table 2].
Table 1: Overall prevalence of methicillin-resistant Staphylococcus aureus.
|
Abbas et al,5 2015
|
201
|
500
|
0.4
|
0.36–0.45
|
240.0
|
1.1
|
|
Agarwal et al,6 2015
|
28
|
96
|
0.29
|
0.20–0.39
|
0.5
|
1
|
|
Agarwala et al,8 2016
|
7
|
1550
|
0
|
0.00–0.01
|
7.6
|
1.1
|
|
Akhtar et al,9 2016
|
87
|
250
|
0.35
|
0.29–0.41
|
1.2
|
1.1
|
|
Ambika et al,10 2017
|
15
|
39
|
0.38
|
0.23–0.55
|
0.2
|
1
|
|
Arunkumar et al,11 2017
|
5
|
100
|
0.05
|
0.02–0.11
|
0.5
|
1
|
|
De Backer et al,12 2019
|
5
|
9
|
0.56
|
0.21–0.86
|
0
|
0.7
|
|
Banerjee et al,13 2018
|
12
|
26
|
0.46
|
0.27–0.67
|
0.1
|
0.9
|
|
Baruah et al,14 2019
|
13
|
190
|
0.07
|
0.04–0.11
|
0.9
|
1
|
|
Bhat et al,15 2016
|
54
|
89
|
0.61
|
0.50–0.71
|
0.4
|
1
|
|
Bhatt et al,16 2015
|
103
|
510
|
0.20
|
0.17–0.24
|
2.5
|
1.1
|
|
Bhattacharya et al,17 2015
|
47
|
100
|
0.47
|
0.37–0.57
|
0.5
|
1
|
|
Bhattacharyya et al,18 2017
|
20
|
122
|
0.16
|
0.10–0.24
|
0.6
|
1
|
|
Bhavana et al,19 2017
|
89
|
200
|
0.44
|
0.37–0.52
|
1
|
1.1
|
|
Bhavana et al,20 2019
|
70
|
187
|
0.37
|
0.30–0.45
|
0.9
|
1
|
|
Bhavsar et al,21 2015
|
65
|
150
|
0.43
|
0.35–0.52
|
0.7
|
1
|
|
Bhowmik et al,22 2019
|
71
|
127
|
0.56
|
0.47–0.65
|
0.6
|
1
|
|
Bhutia et al,23 2015
|
53
|
150
|
0.35
|
0.28–0.44
|
0.7
|
1
|
|
Bouchiat et al,24 2015
|
48
|
92
|
0.52
|
0.42–0.63
|
0.4
|
1
|
|
Chaudhary et al,25 2015
|
77
|
178
|
0.43
|
0.36–0.51
|
0.9
|
1
|
|
Choudhury et al,26 2016
|
311
|
724
|
0.43
|
0.39–0.47
|
3.5
|
1.1
|
|
Cugati et al,27 2017
|
92
|
161
|
0.57
|
0.49–0.65
|
0.8
|
1
|
|
Dass et al,28 2016
|
64
|
100
|
0.64
|
0.54–0.73
|
0.5
|
1
|
|
Datta et al,29 2019
|
5
|
26
|
0.19
|
0.07–0.39
|
0.1
|
0.9
|
|
Deepika et al,30 2015
|
25
|
29
|
0.86
|
0.68–0.96
|
0.1
|
0.9
|
|
Dhiman et al,31 2017
|
24
|
150
|
0.16
|
0.11–0.23
|
0.7
|
1
|
|
Dixit,32 2018
|
21
|
42
|
0.5
|
0.34–0.66
|
0.2
|
1
|
|
Farooq et al,33 2016
|
210
|
343
|
0.61
|
0.56–0.66
|
1.7
|
1.1
|
|
Geetha et al,34 2015
|
44
|
166
|
0.27
|
0.20–0.34
|
0.8
|
1
|
|
Ghosh et al,35 2016
|
11
|
46
|
0.24
|
0.13–0.39
|
0.2
|
1
|
|
Govindan et al,36 2015
|
17
|
441
|
0.04
|
0.02–0.06
|
2.2
|
1.1
|
|
Gupta and Sinha,37 2017
|
344
|
450
|
0.76
|
0.72–0.80
|
2.2
|
1.1
|
|
Gupta et al,38 2015a
|
19
|
60
|
0.32
|
0.20–0.45
|
0.3
|
1
|
|
Gupta et al,39 2015b
|
12
|
30
|
0.4
|
0.23–0.59
|
0.1
|
0.9
|
|
Gupta et al,40 2016
|
69
|
174
|
0.4
|
0.32–0.47
|
0.8
|
1
|
|
Gupta et al,41 2017
|
408
|
505
|
0.81
|
0.77–0.84
|
2.5
|
1.1
|
|
Hemamalini et al,42 2015
|
14
|
40
|
0.35
|
0.21–0.52
|
0.2
|
1
|
|
Hussain et al,43 2015
|
53
|
80
|
0.66
|
0.55–0.76
|
0.4
|
1
|
|
Jana et al,44 2015
|
23
|
122
|
0.19
|
0.12–0.27
|
0.6
|
1
|
|
Jindal et al,45 2016
|
161
|
248
|
0.65
|
0.59–0.71
|
1.2
|
1.1
|
|
John et al,46 2019
|
18
|
100
|
0.18
|
0.11–0.27
|
0.5
|
1
|
|
Joshi et al,47 2017
|
34
|
231
|
0.15
|
0.10–0.20
|
1.1
|
1.1
|
|
Kaur et al,48 2019
|
83
|
162
|
0.51
|
0.43–0.59
|
0.8
|
1
|
|
Kavitha et al,49 2017
|
22
|
207
|
0.11
|
0.07–0.16
|
1
|
1.1
|
|
Kogekar et al,50 2015
|
16
|
30
|
0.53
|
0.34–0.72
|
0.1
|
0.9
|
|
Kulshrestha et al,51 2017
|
82
|
161
|
0.51
|
0.43–0.59
|
0.8
|
1
|
|
Kulshrestha et al,52 2019
|
73
|
214
|
0.34
|
0.28–0.41
|
1
|
1.1
|
|
Kumar et al,53 2016
|
79
|
147
|
0.54
|
0.45–0.62
|
0.7
|
1
|
|
Kumari et al,54 2016
|
88
|
291
|
0.3
|
0.25–0.36
|
1.4
|
1.1
|
|
Majhi et al,55 2016
|
129
|
209
|
0.62
|
0.55–0.68
|
1
|
1.1
|
|
Mamtora et al,56 2019
|
310
|
1041
|
0.3
|
0.27–0.33
|
5.1
|
1.1
|
|
Mehta,57 2017
|
145
|
250
|
0.58
|
0.52–0.64
|
1.2
|
1.1
|
|
Mendem et al,58 2016
|
24
|
62
|
0.39
|
0.27–0.52
|
0.3
|
1
|
|
Mohanty et al,59 2019
|
127
|
284
|
0.45
|
0.39–0.51
|
1.4
|
1.1
|
|
Mokta et al,60 2015
|
82
|
350
|
0.23
|
0.19–0.28
|
1.7
|
1.1
|
|
Mondal et al,61 2016
|
16
|
87
|
0.18
|
0.11–0.28
|
0.4
|
1
|
|
Mundhada et al,62 2017
|
14
|
112
|
0.12
|
0.07–0.20
|
0.5
|
1
|
|
Mushtaq et al,632016
|
58
|
140
|
0.41
|
0.33–0.50
|
0.7
|
1
|
|
Nadimpalli et al,64 2016
|
63
|
2040
|
0.03
|
0.02–0.04
|
10
|
1.1
|
|
Nagamadhavi et al,65 2016
|
2
|
91
|
0.02
|
0.00–0.08
|
0.4
|
1
|
|
Nagaraju et al,66 2017
|
41
|
274
|
0.15
|
0.11–0.20
|
1.3
|
1.1
|
|
Nagasundaram et al,67 2019
|
114
|
200
|
0.57
|
0.50–0.64
|
1
|
1.1
|
|
Negi et al,68 2015
|
11
|
70
|
0.16
|
0.08–0.26
|
0.3
|
1
|
|
Pai et al,69 2015
|
7
|
33
|
0.21
|
0.09–0.39
|
0.2
|
0.9
|
|
Pai et al,70 2017
|
9
|
100
|
0.09
|
0.04–0.16
|
0.5
|
1
|
|
Pal et al,71 2019
|
34
|
121
|
0.28
|
0.20–0.37
|
0.6
|
1
|
|
Pandya et al,72 2015
|
104
|
180
|
0.58
|
0.50–0.65
|
0.9
|
1
|
|
Patil et al,73 2017
|
23
|
57
|
0.4
|
0.28–0.54
|
0.3
|
1
|
|
Patil et al,74 2019
|
11
|
47
|
0.23
|
0.12–0.38
|
0.2
|
1
|
|
Perala et al,75 2016
|
132
|
386
|
0.34
|
0.29–0.39
|
1.9
|
1.1
|
|
Perween et al,76 2015
|
80
|
141
|
0.57
|
0.48–0.65
|
0.7
|
1
|
|
Phukan et al,77 2015
|
160
|
215
|
0.74
|
0.68–0.80
|
1
|
1.1
|
|
Radhakrishna et al,78 2016
|
9
|
78
|
0.12
|
0.05–0.21
|
0.4
|
1
|
|
Raigar et al,79 2019
|
208
|
400
|
0.52
|
0.47–0.57
|
2
|
1.1
|
|
Rana-Khara et al,80 2016
|
52
|
100
|
0.52
|
0.42–0.62
|
0.5
|
1
|
|
Reema et al,81 2016
|
23
|
50
|
0.46
|
0.32–0.61
|
0.2
|
1
|
|
Rengaraj et al,82 2016
|
54
|
109
|
0.5
|
0.40–0.59
|
0.5
|
1
|
|
Routray et al,83 2019
|
13
|
17
|
0.76
|
0.50–0.93
|
0.1
|
0.9
|
|
Roy,84 2018
|
9
|
38
|
0.24
|
0.11–0.40
|
0.2
|
1
|
|
Rudresh et al,85 2015
|
22
|
98
|
0.22
|
0.15–0.32
|
0.5
|
1
|
|
Sankaran et al,86 2018
|
13
|
30
|
0.43
|
0.25–0.63
|
0.1
|
0.9
|
|
Selvabai et al,87 2019
|
114
|
468
|
0.24
|
0.21–0.29
|
2.3
|
1.1
|
|
Sengupta et al,88 2016
|
19
|
19
|
1
|
0.82–1.00
|
0.1
|
0.9
|
|
Senthilkumar et al,89 2015
|
46
|
98
|
0.47
|
0.37–0.57
|
0.5
|
1
|
|
Shinde et al,90 2016
|
9
|
26
|
0.35
|
0.17–0.56
|
0.1
|
0.9
|
|
Singh et al,91 2017
|
15
|
200
|
0.08
|
0.04–0.12
|
1
|
1.1
|
|
Singh et al,92 2018
|
87
|
248
|
0.35
|
0.29–0.41
|
1.2
|
1.1
|
|
Singh et al,93 2018
|
9
|
49
|
0.18
|
0.09–0.32
|
0.2
|
1
|
|
Swathirajan et al,94 2020
|
262
|
380
|
0.69
|
0.64–0.74
|
1.9
|
1.1
|
|
Talwar et al,95 2016
|
38
|
111
|
0.34
|
0.25–0.44
|
0.5
|
1
|
|
There et al,96 2016
|
50
|
114
|
0.44
|
0.35–0.53
|
0.6
|
1
|
|
Thomas et al,97 2018
|
14
|
43
|
0.33
|
0.19–0.49
|
0.2
|
1
|
|
Tiewsoh et al,98 2017
|
24
|
432
|
0.06
|
0.04–0.08
|
2.1
|
1.1
|
|
Tripathi,99 2015
|
70
|
210
|
0.33
|
0.27–0.40
|
1
|
1.1
|
|
Trivedi et al,100 2015
|
47
|
232
|
0.2
|
0.15–0.26
|
1.1
|
1.1
|
|
Vasuki et al,101 2016
|
45
|
83
|
0.54
|
0.43–0.65
|
0.4
|
1
|
|
Velayudham et al,102 2017
|
120
|
182
|
0.66
|
0.59–0.73
|
0.9
|
1
|
|
Venkatesan et al,1032017
|
23
|
43
|
0.53
|
0.38–0.69
|
0.2
|
1
|
|
Fixed effect model
|
|
20493
|
0.29
|
0.28–0.29
|
100%
|
_____
|
Heterogeneity: I2 = 99%, τ2 = 0.0571, p < 0.001.
Table 2: Details of pooled prevalence of methicillin-resistant Staphylococcus aureus in 22 districts during 2015–2020.
|
1
|
Andhra Pradesh
|
37 (0–89)
|
98
|
0.2642
|
< 0.01
|
|
2
|
Assam
|
43 (15–74)
|
99
|
0.1071
|
< 0.01
|
|
3
|
Gujarat
|
46 (31–60)
|
96
|
0.0268
|
< 0.01
|
|
4
|
Haryana
|
35 (31–39)
|
0
|
0
|
0.95
|
|
5
|
Himachal Pradesh
|
27 (13–44)
|
94
|
0.0229
|
< 0.01
|
|
6
|
Jammu and Kashmir
|
55 (42–67)
|
88
|
0.0112
|
< 0.01
|
|
7
|
Karnataka
|
23 (14–33)
|
96
|
0.0399
|
< 0.01
|
|
8
|
Kerala
|
30 (16–45)
|
77
|
0.0156
|
0.01
|
|
9
|
Madhya Pradesh
|
36 (25–47)
|
78
|
0.0112
|
< 0.01
|
|
10
|
Maharashtra
|
21 (11–34)
|
99
|
0.0517
|
< 0.01
|
|
11
|
New Delhi
|
52 (32–71)
|
89
|
0.0288
|
< 0.01
|
|
12
|
Odisha
|
49 (25–73)
|
93
|
0.0599
|
< 0.01
|
|
13
|
Puducherry
|
44 (19–70)
|
98
|
0.0730
|
< 0.01
|
|
14
|
Punjab
|
37 (16–61)
|
98
|
0.0738
|
< 0.01
|
|
15
|
Rajasthan
|
48 (42–54)
|
77
|
0.0031
|
< 0.01
|
|
16
|
Sikkim*
|
35 (28–44)
|
-
|
-
|
-
|
|
17
|
Tamil Nadu
|
44 (29–60)
|
97
|
0.0544
|
< 0.01
|
|
18
|
Telangana
|
38 (20–58)
|
66
|
0.0202
|
0.05
|
|
19
|
Tripura
|
36 (15–60)
|
85
|
0.0260
|
< 0.01
|
|
20
|
Uttar Pradesh
|
53 (30–75)
|
98
|
0.0670
|
< 0.01
|
|
21
|
Uttarakhand
|
26 (16–37)
|
76
|
0.0089
|
0.02
|
*Single article.

 Figure 2: Heterogeneity assessment.
Figure 2: Heterogeneity assessment.
Table 3: Year-wise prevalence of methicillin-resistant Staphylococcus aureus in India during 2015–2020.
|
2015
|
38 (30–45)
|
97
|
0.0414
|
< 0.01
|
|
2016
|
39 (29–50)
|
99
|
0.0797
|
< 0.01
|
|
2017
|
31 (20–44)
|
99
|
0.0835
|
< 0.01
|
|
2018
|
35 (26–43)
|
62
|
0.0091
|
0.02
|
|
2019
|
37 (28–46)
|
95
|
0.0343
|
< 0.01
|
*Single article
Table 4: Zone-wise prevalence of methicillin-resistant Staphylococcus aureus in India during 2015–2020.
|
1
|
North
(Uttar Pradesh, Haryana, Jammu and Kashmir, Himachal Pradesh, Punjab, New Delhi, and Uttarakhand)
|
41 (33–50)
|
98
|
0.0446
|
991.31
|
< 0.01
|
-1.55
|
0.14
|
1000.57
|
|
2
|
South
(Tamil Nadu, Telangana, Karnataka Andhra Pradesh, Kerala, and Puducherry)
|
34 (26–42)
|
98
|
0.0614
|
1351.91
|
< 0.01
|
1.19
|
0.24
|
1369.91
|
|
3
|
West
(Rajasthan, Maharashtra, and Gujarat)
|
33 (24–43)
|
99
|
0.0514
|
2551.24
|
< 0.001
|
2.3
|
0.030
|
2559.54
|
|
4
|
East
(West Bengal and Odisha)
|
43 (20–68)
|
96
|
0.01401
|
193.14
|
< 0.01
|
0.57
|
0.58
|
209.95
|
|
5
|
North East
(Assam, Tripura, and Sikkim)
|
40 (23–58)
|
98
|
0.0601
|
260.52
|
< 0.01
|
-0.27
|
0.8
|
264.06
|
|
6
|
Central
(Madhya Pradesh)
|
36 (25–47)
|
78
|
0.0112
|
13.3
|
< 0.01
|
0.58
|
0.62
|
13.54
|

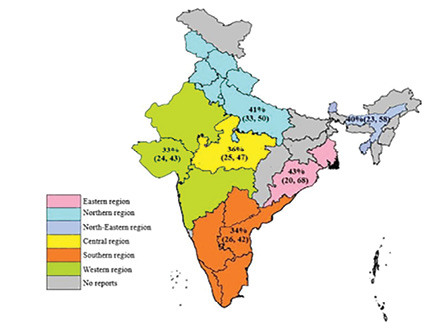 Figure 3: Zone analysis.
Figure 3: Zone analysis.

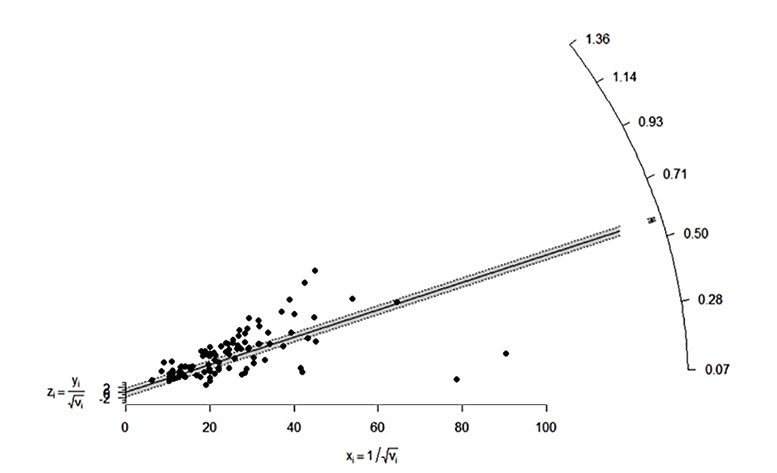 Figure 4: Galbraith plot assessment between study reports.
Figure 4: Galbraith plot assessment between study reports.
Table 5: Test for residual heterogeneity.
|
1
|
Year
|
0.00
|
0.0577
|
97.91
|
47.78
|
0.0039
|
0.950
|
|
2
|
Sample size
|
7.03
|
0.0531
|
97.61
|
41.79
|
7.8623
|
0.005
|
|
3
|
Region
|
0.00
|
0.0588
|
97.89
|
47.29
|
2.3638
|
0.796
|
Table 6: Meta-regression parameter estimate.
|
1
|
Year
|
-0.0011
|
-0.0354–0.0332
|
0.935
|
|
2
|
Sample size
|
-0.0002
|
-0.0004–-0.0001
|
0.005
|
|
Group I (more than median)
|
|
0.5810–0.7210
|
3.744778e-75
|
|
Group II (less than median)
|
|
0.5840–0.7200
|
1.910528e-78
|
|
3
|
Region
|
|
|
|
|
Central
|
Reference
|
|
|
|
East
|
0.0592
|
-0.2354–0.3537
|
0.693
|
|
North
|
0.0482
|
-0.2151–0.3116
|
0.719
|
|
Northeast
|
0.0339
|
-0.2711–0.3389
|
0.827
|
|
South
|
-0.0349
|
-0.2927–0.2228
|
0.790
|
|
West
|
-0.0221
|
-0.2901–0.2459
|
0.871
|
|
4
|
Confirmatory test
|
|
|
|
|
MeReSa agar screening
|
Reference
|
|
|
|
Double disk diffusion erythromycin and clindamycin
|
0.54
|
0.0499–1.0302
|
0.060
|
|
Kirby Bauer disk diffusion method Cefoxitin
|
0.1621
|
-0.0036–0.3278
|
0.055
|
Year-wise prevalence of MRSA
Heterogeneity assessment was performed year-wise [Figure 2]. It was found that the studies published in 2015, 2016, 2017, 2018, and 2019 have independent significant heterogeneity; hence subgroup analysis is more appropriate using the random effect model to deal with heterogeneity.
In 2015, 27 articles showed the prevalence of MRSA as 38% (95% CI: 30–45) with I2 = -97, τ2 = -0.0414, p < 0.01. In 2016, 27 articles showed the prevalence of MRSA as 39% (95% CI: 29–50) with I2 = -99, τ2 = -0.0797, p < 0.01. In 2017, 20 articles showed the prevalence of MRSA as 31% (95% CI: 20–44) with I2 = -99, τ2 = -0.0835, p < 0.001. In 2018, 7 articles showed the prevalence of MRSA as 35% (95% CI: 26–43) with I2 = -62, τ2 = -0.0091, p = 0.02. In 2019, 16 articles showed the prevalence of MRSA as 37% (95% CI: 28–46) with I2 = -95, τ2 = -0.0343, p < 0.01. In 2020, a single article showed prevalence of MRSA as 69% (95% CI: 64–74) [Table 3].
Zone-wise prevalence of MRSA
In zone-wise analysis [Table 4 and Figure 3], the east zone with nine articles (West Bengal and Odisha) showed the highest pooled prevalence of 43% (95% CI: 20–68) with I2 = -96, τ2 = 0.01401, p < 0.01. The lowest prevalence of MRSA was recorded in the west zone with 20 articles (Rajasthan, Maharashtra, and Gujarat) as 33% (95% CI: 24–43) with I2 = -99, τ2 = -0.0514, p < 0.001, and these states are geographically large and densely populated. Twenty-four articles in the north zone comprising Uttara Pradesh, Haryana, Jammu and Kashmir, Himachal Pradesh, Punjab, New Delhi, and Uttarakhand had a pooled prevalence of 41% (95% CI: 33–50) with I2 = -98, τ2 = -0.0446, p < 0.01. Thirty-four articles in the south zone consisting of Tamil Nadu, Telangana, Karnataka, Andhra Pradesh, Kerala, and Puducherry revealed a pooled prevalence of MRSA as 34% (95% CI: 26–42) with I= -98, τ2 = -0.0614, p < 0.01. Four articles in central zone (Madhya Pradesh) showed a pooled prevalence of 36% (95% CI: 25–47) with I2 = -78, τ2 = -0.0112, p < 0.01. Assam, Tripura, and Sikkim are part of the northeast zone (seven articles) which showed a pooled prevalence of MRSA as 40% (95% CI: 23–58) with I2 = -98, τ2 = -0.0601, p < 0.01.
Meta-regression analysis
Meta-regression is a tool used to examine the effect of moderators on MRSA prevalence rates. In this study, the year of publications, sample size, geographical regions, and confirmatory tests used for the diagnosis of samples are the moderators. After conducting the meta-regression, sample size was found significant (R2 = 7.03; p = 0.005). The heterogeneity contribution of the moderator variables ranged from 0 to 7.03%. Further investigation of subgroup analysis of sample size was performed, dividing the sample size moderator into two groups viz., less than median and more than median, using a mixed-effect model, which yielded I2 = 99%, p = 0.990. The results of the tests for residual heterogeneity and parameter estimation by meta-regression are presented in Tables 5 and 6.
The study included 74 hospitals and 24 community settings (total of 98 articles). Further investigation of subgroup analysis of hospital and community settings was conducted. The pooled prevalence of MRSA for community settings was 27% (95% CI: 19–35) (I2 = -96, τ2 = -0.0521, p < 0.01) and that for hospital setting was 49% (95% CI: 35–45) (I2 = -99, τ2 = -0.0542, p < 0.001) [Table 7].
To assess the heterogeneity between study reports, we generated a Galbraith plot [Figure 4]. The standardized effect estimates against inverse standard error were shown as scattered points in the plot. The points representing the study reports outside confidence bounds may be contributing to the heterogeneity. In the absence of heterogeneity, all points (reports) are expected to lie within the confidence limits centering around the line.
Table 7: Pooled prevalence of methicillin-resistant Staphylococcus aureus in community settings.
|
Community
|
|
|
|
|
|
|
Abbas et al,5 2015
|
201
|
500
|
0.4
|
0.36–0.45
|
1.1
|
|
Agarwal et al,6 2015
|
28
|
96
|
0.29
|
0.20–0.39
|
1
|
|
Ambika et al,10 2017
|
15
|
39
|
0.38
|
0.23–0.55
|
1
|
|
Banerjee et al,13 2019
|
12
|
26
|
0.46
|
0.27–0.67
|
0.9
|
|
Bhavana et al,19 2017
|
89
|
200
|
0.44
|
0.37–0.52
|
1.1
|
|
Bhutia et al,23 2015
|
53
|
150
|
0.35
|
0.28–0.44
|
1
|
|
Bouchiat et al,24 2015
|
48
|
92
|
0.52
|
0.42–0.63
|
1
|
|
Deepika et al,30 2015
|
25
|
29
|
0.86
|
0.34–0.66
|
0.9
|
|
Dixit,32 2018
|
21
|
42
|
0.5
|
0.68–0.96
|
1
|
|
Govindan et al,36 2015
|
17
|
441
|
0.04
|
0.02–0.06
|
1.1
|
|
Jana et al,44 2015
|
23
|
122
|
0.19
|
0.12–0.27
|
1
|
|
John et al,46 2019
|
18
|
100
|
0.18
|
0.11–0.27
|
1
|
|
Kogekar et al,50 2015
|
16
|
30
|
0.53
|
0.34–0.72
|
0.9
|
|
Kulshrestha et al,51 2017
|
73
|
214
|
0.34
|
0.43–0.59
|
1.1
|
|
Mondal et al,61 2016
|
16
|
87
|
0.18
|
0.11–0.28
|
1
|
|
Mundhada et al,62 2017
|
14
|
112
|
0.12
|
0.07–0.20
|
1
|
|
Nagamadhavi et al,65 2016
|
2
|
91
|
0.02
|
0.00–0.08
|
1
|
|
Nagaraju et al,66 2017
|
41
|
274
|
0.15
|
0.11–0.20
|
1.1
|
|
Patil et al,74 2019
|
11
|
47
|
0.23
|
0.12–0.38
|
1
|
|
Radhakrishna et al,78 2016
|
9
|
78
|
0.12
|
0.05–0.21
|
1
|
|
Roy,84 2018
|
9
|
38
|
0.24
|
0.11–0.40
|
1
|
|
Shinde et al,90 2016
|
9
|
26
|
0.35
|
0.17–0.56
|
0.9
|
|
Singh et al,91 2017
|
15
|
200
|
0.08
|
0.04–0.12
|
1.1
|
|
Tiewsoh and Dias,98 2017
|
24
|
432
|
0.06
|
0.04–0.08
|
1.1
|
|
Random effects model
|
|
|
0.27
|
0.19–0.5
|
24.2
|
|
Heterogeneity : I2 = 99%, τ2 = 0.0521, p = 0.01
|
|
Hospital
|
|
|
|
|
|
|
Agarwala et al,8 2016
|
7
|
1550
|
0
|
0.00–0.01
|
1.1
|
|
Akhtar et al,9 2016
|
87
|
250
|
0.35
|
0.29–0.41
|
1.1
|
|
Arunkumar et al,11 2017
|
5
|
100
|
0.05
|
0.02–0.11
|
1
|
|
De Backer et al,12 2019
|
5
|
9
|
0.56
|
0.21–0.86
|
0.7
|
|
Baruah et al,14 2019
|
13
|
190
|
0.07
|
0.04–0.11
|
1
|
|
Bhat et al,15 2016
|
54
|
89
|
0.61
|
0.50–0.71
|
1
|
|
Bhatt et al,16 2015
|
103
|
510
|
0.2
|
0.17–0.24
|
1.1
|
|
Bhattacharya et al,17 2015
|
47
|
100
|
0.47
|
0.37–0.57
|
1
|
|
Bhattacharyya et al,18 2017
|
20
|
122
|
0.16
|
0.10–0.24
|
1
|
|
Bhavana et al,20 2019
|
70
|
187
|
0.37
|
0.30–0.45
|
1
|
|
Bhavsar et al,21 2015
|
65
|
150
|
0.43
|
0.35–0.52
|
1
|
|
Bhowmik et al,22 2019
|
71
|
127
|
0.56
|
0.47–0.65
|
1
|
|
Chaudhary et al,25 2015
|
77
|
178
|
0.43
|
0.36–0.51
|
1
|
|
Choudhury et al,26 2016
|
311
|
724
|
0.43
|
0.39–0.47
|
1.1
|
|
Cugati et al,27 2017
|
92
|
161
|
0.57
|
0.49–0.65
|
1
|
|
Dass et al,28 2016
|
64
|
100
|
0.64
|
0.54–0.73
|
1
|
|
Datta et al,29 2019
|
5
|
26
|
0.19
|
0.07–0.39
|
0.9
|
|
Dhiman et al,31 2017
|
24
|
150
|
0.16
|
0.11–0.23
|
1
|
|
Farooq et al,33 2016
|
210
|
343
|
0.61
|
0.56–0.66
|
1.1
|
|
Geetha et al,34 2015
|
44
|
166
|
0.27
|
0.20–0.34
|
1
|
|
Ghosh et al,35 2016
|
11
|
46
|
0.24
|
0.13–0.39
|
1
|
|
Gupta et al,37 2017
|
344
|
450
|
0.76
|
0.72–0.80
|
1.1
|
|
Gupta et al,38 2015
|
19
|
60
|
0.32
|
0.20–0.45
|
1
|
|
Gupta et al,39 2015
|
12
|
30
|
0.4
|
0.23–0.59
|
0.9
|
|
Gupta et al,40 2016
|
69
|
174
|
0.4
|
0.32–0.47
|
1
|
|
Gupta et al,41 2017
|
408
|
505
|
0.81
|
0.77–0.84
|
1.1
|
|
Hemamalini et al,42 2015
|
14
|
40
|
0.35
|
0.21–0.52
|
1
|
|
Hussain et al,43 2015
|
53
|
80
|
0.66
|
0.55–0.76
|
1
|
|
Jindal et al,45 2016
|
161
|
248
|
0.65
|
0.59–0.71
|
1.1
|
|
Joshi et al,47 2017
|
34
|
231
|
0.15
|
0.10–0.20
|
1.1
|
|
Kaur et al,48 2019
|
83
|
162
|
0.51
|
0.43–0.59
|
1
|
|
Kavitha et al,49 2017
|
22
|
207
|
0.11
|
0.07–0.16
|
1.1
|
|
Kulshrestha et al,52 2019
|
82
|
161
|
0.51
|
0.28–0.41
|
1
|
|
Kumar et al,53 2016
|
79
|
147
|
0.54
|
0.45–0.62
|
1
|
|
Kumari et al,54 2016
|
88
|
291
|
0.3
|
0.25–0.36
|
1.1
|
|
Majhi et al,55 2016
|
129
|
209
|
0.62
|
0.55–0.68
|
1.1
|
|
Mamtora et al,56 2019
|
310
|
1041
|
0.3
|
0.27–0.33
|
1.1
|
|
Mehta,57 2017
|
145
|
250
|
0.58
|
0.52–0.64
|
1.1
|
|
Mendem et al,58 2016
|
24
|
62
|
0.39
|
0.27–0.52
|
1
|
|
Mohanty et al,59 2019
|
127
|
284
|
0.45
|
0.39–0.51
|
1.1
|
|
Mokta et al,60 2015
|
82
|
350
|
0.23
|
0.19–0.28
|
1.1
|
|
Mushtaq et al,63 2016
|
58
|
140
|
0.41
|
0.33–0.50
|
1
|
|
Nadimpalli et al,64 2016
|
63
|
2040
|
0.03
|
0.02–0.04
|
1.1
|
|
Nagasundaram et al,67 2019
|
114
|
200
|
0.57
|
0.50–0.64
|
1.1
|
|
Negi et al,68 2015
|
11
|
70
|
0.16
|
0.08–0.26
|
1
|
|
Pai et al,69 2015
|
7
|
33
|
0.21
|
0.09–0.39
|
0.9
|
|
Pai et al,70 2017
|
9
|
100
|
0.09
|
0.04–0.16
|
1
|
|
Pal et al,71 2019
|
34
|
121
|
0.28
|
0.20–0.37
|
1
|
|
Pandya et al,72 2015
|
104
|
180
|
0.58
|
0.50–0.65
|
1
|
|
Patil et al,73 2017
|
23
|
57
|
0.4
|
0.28–0.54
|
1
|
|
Perala et al,75 2016
|
132
|
386
|
0.34
|
0.29–0.39
|
1.1
|
|
Perween et al,76 2015
|
80
|
141
|
0.57
|
0.48–0.65
|
1
|
|
Phukan et al,77 2015
|
160
|
215
|
0.74
|
0.68–0.80
|
1.1
|
|
Raigar et al,79 2019
|
208
|
400
|
0.52
|
0.47–0.57
|
1.1
|
|
Rana-Khara et al,80 2016
|
52
|
100
|
0.52
|
0.42–0.62
|
1
|
|
Reema et al,81 2016
|
23
|
50
|
0.46
|
0.32–0.61
|
1
|
|
Rengaraj et al,82 2016
|
54
|
109
|
0.5
|
0.40–0.59
|
1
|
|
Routray et al,83 2019
|
13
|
17
|
0.76
|
0.50–0.93
|
0.9
|
|
Rudresh et al,85 2015
|
22
|
98
|
0.22
|
0.15–0.32
|
1
|
|
Sankaran et al,86 2018
|
13
|
30
|
0.43
|
0.25–0.63
|
0.9
|
|
Selvabai et al,87 2019
|
114
|
468
|
0.24
|
0.21–0.29
|
1.1
|
|
Sengupta et al,88 2016
|
19
|
19
|
1
|
0.82–1.00
|
0.9
|
|
Senthilkumar et al,89 2015
|
46
|
98
|
0.47
|
0.37–0.57
|
1
|
|
Singh et al,92 2018
|
87
|
248
|
0.35
|
0.29–0.41
|
1.1
|
|
Singh et al,93 2018
|
9
|
49
|
0.18
|
0.09–0.32
|
1
|
|
Swathirajan et al,94 2020
|
262
|
380
|
0.69
|
0.64–0.74
|
1.1
|
|
Talwar et al,95 2016
|
38
|
111
|
0.34
|
0.25–0.44
|
1
|
|
There et al,96 2016
|
50
|
114
|
0.44
|
0.35–0.53
|
1
|
|
Thomas et al,97 2018
|
14
|
43
|
0.33
|
0.19–0.49
|
1
|
|
Tripathi,99 2015
|
70
|
210
|
0.33
|
0.27–0.40
|
1.1
|
|
Trivedi et al,100 2015
|
47
|
232
|
0.2
|
0.15–0.26
|
1.1
|
|
Vasuki et al,101 2016
|
45
|
83
|
0.54
|
0.43–0.65
|
1
|
|
Velayudham et al,102 2017
|
120
|
182
|
0.66
|
0.59–0.73
|
1
|
|
Venkatesan et al,103 2017
|
23
|
43
|
0.53
|
0.38–0.69
|
1
|
|
Random effects model
|
|
17027
|
0.4
|
0.35–0.45
|
75.8
|
|
Heterogeneity: I2 = 99%, τ2 = 0.0542, p < 0.001
|
|
Random effects model
|
|
20493
|
0.37
|
0.32–0.41
|
100
|
|
Heterogeneity: I2 = 99%, τ2 =0.0571, p < 0.001
|
Discussion
Antibiotic resistance is one of the foremost health concerns of India. There has been an alarming increase in the prevalence of S. aureus resistant to methicillin in India in recent years, especially community-associated MRSA. MRSA is now endemic in India, and its incidence is varied. The current policy shows a growing political commitment at the highest levels to take strong action on antimicrobial resistance and provide adequate support for nationwide surveillance and stewardship to mitigate the resistance problem.80
Our meta-analysis study reveals the pooled prevalence of MRSA in India at 37% (95% CI: 32–41) during 2015–2020. The epidemiology of MRSA in humans is changing gradually in India and the prevalence has increased over the years due to lack of awareness, overuse of antimicrobial medicines in human health, increase in the infections caused due to lack of sanitation and hygiene, and the paucity of stringent rules and regulations for use of antibiotics. Although the cost of antibiotics is high, the consumption rate has increased due to inappropriate prescribing, indiscriminate use of antibiotics, and sales of antibiotics without prescription. Self-medication with antibiotics bought without prescription is also a serious concern in India.
A pooled prevalence of MRSA varied between 31%–39% from 2015 to 2019 (69% in 2020) against a total prevalence of 37% across India. Jammu and Kashmir showed the highest prevalence of MRSA (55%), which shares a border with Pakistan, though illegal movement may not be ruled out alongside borders. On the other hand, Maharashtra has the lowest prevalence of MRSA (21%) and has more sophisticated hospitals.
In zone-wise analysis, the east zone has shown the highest prevalence of MRSA (43%), including West Bengal and Odisha. West Bengal shares a porous border with Bangladesh, and there is no restriction on the movement of men and material between them. The north zone, which included Uttar Pradesh, Haryana, Jammu and Kashmir, Himachal Pradesh, Punjab, New Delhi, and Uttarakhand states, had the second-highest (41%) MRSA prevalence. The northeast zone, which comprises Assam, Tripura, and Sikkim, has shown the third-highest prevalence of MRSA (40%). Assam has a porous border with Bhutan and Bangladesh; Tripura shares a porous border with Bangladesh whereas Sikkim shares with Bhutan, Tibet, and Nepal. There is no restriction on the movement of men and materials. In a similar study,104 46% and 54% of prevalence of MRSA among females and males, respectively, was recorded in the west zone of Iran. Eighty-four isolates from the intensive care unit of a hospital in Iran were antimicrobial-resistant, which is quite alarming.105
In year-wise analysis, the pooled prevalence of MRSA was more (39%) during 2016, followed by 38% prevalence in 2015. The reports on the prevalence of MRSA (35%) were more homogenous (I2 = 62%). There was a consistency in reporting of prevalence rate of MRSA in all zones of India.
The moderate heterogeneity may be due to the size’s total variability effect, which might not have been caused by sampling error. Further, the heterogeneity between studies can be attributed to the different study settings and study populations since the studies on MRSA prevalence from different regions are limited. Heterogeneity between studies could also be due to different population settings under investigation, type of samples used, geographical locations, and hospital/community practices. However, the weight (fixed) assigned to 24 studies under community settings did not exhibit outlier features upon scrutinizing the forest plots. Therefore, the effect of two settings (hospital and community) on pooled prevalence of MRSA was not found to have a large difference. The subgroup analysis of studies revealed that the pooled prevalence of MRSA in the hospital setting was 49% and 27% in the community setting.
Further to meta-analysis, barring selection bias, systematic reviews helps the revision of all the scientific evidence on a given topic. Based on the output, the summarized information can be used to propose hypotheses that explain the data’s behavior and identify areas of gaps where further research is needed.106 However, it is a controversial tool because several conditions are critical, and even small violations of these can lead to misleading conclusions. While designing and performing a meta-analysis, several decisions concerning personal judgment and expertise need to be made that may eventually create bias or expectations that influence the result.107
Conclusion
The overall pooled prevalence of MRSA in India was very high (37%). Studies comprising large populations in different locations with rapid tests would be of much help in computing the prevalence of MRSA. This increase in the prevalence of MRSA builds more emphasis on the need to develop more stringent policies and regulations for the use of antibiotics in the human healthcare system. Strict adherence to hand hygiene and judicious use of any antibiotics will greatly reduce the incidence of MRSA. Awareness of the indiscriminate use of antibiotics and preventive strategies should be introduced to combat the epidemic spread of drug- resistant bacteria in India.
Disclosure
The authors declared no conflicts of interest.
Acknowledgments
The authors would like to thank the Director of the institute, DG, ICAR, DDG (AS), and ICAR for their support and guidance throughout the study. Special thanks to Ms. K N Mandara typesetting.
references
- 1. Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008 Mar;19(2):173-184.
- 2. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015 Jul;28(3):603-661.
- 3. Sasirekha B, Usha MS, Amruta AJ, Ankit S, Brinda
N, Divya R. Evaluation and comparison of different phenotypic tests to detect methicillin resistant Staphylococcus aureus and their biofilm production. Int J Pharm Tech Res 2012;4(2):532-534.
- 4. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T 2015 Apr;40(4):277-283.
- 5. Abbas A, Nirwan PS, Srivastava P. Prevalence and antibiogram of hospital acquired-methicillin resistant Staphylococcus aureus and community acquired-methicillin resistant Staphylococcus aureus at a tertiary care hospital National Institute of Medical Sciences. Communit Acquir Infect 2015;2(1):13.
- 6. Agarwal L, Singh AK, Sengupta C, Agarwal A. Nasal carriage of Methicillin- and Mupirocin-resistant S. aureus among health care workers in a tertiary care hospital. J Res Pharm Pract 2015 Oct-Dec;4(4):182-186.
- 7. Kuralayanapalya SP, Patil SS, Hamsapriya S. Amachwadi, prevalence of extended-spectrum beta-lactamase producing bacteria from animal origin: a systematic review and meta-analysis report from India. PLoS One 2015;14(9):e0221771.
- 8. Agarwala S, Lad D, Agashe V, Sobti A. Prevalence of MRSA colonization in an adult urban Indian population undergoing orthopaedic surgery. J Clin Orthop Trauma 2016 Jan-Mar;7(1):12-16.
- 9. Akhtar S, Chattopadhyay D, Sharma M. Detection of methicillin-resistant Staphylococcus aureus in a rural hospital of Haryana by phenotypic and genotypic methods. Ind J of Health Sci and Care 2016;3(3):110-115.
- 10. Ambika B, Sony Y, Nidhi P, Ashish C. Prevalence of carriage of methicillin resistance Staphylococcus aureus among health care workers in a tertiary care center, Kanpur (U.P). Int J Curr Microbiol Appl Sci 2017;6(5):739-746.
- 11. Arunkumar KV, Prabagaravarthanan R, Bhaskar M. Prevalence of Methicillin-resistant Staphylococcus aureus (MRSA) infections among patients admitted in critical care units in a tertiary care hospital. Int J Res Med Sci 2017;5(6):2362-2366.
- 12. De Backer S, Xavier BB, Vanjari L, Coppens J, Lammens C, Vemu L, et al. Remarkable geographical variations between India and Europe in carriage of the staphylococcal surface protein-encoding sasX/sesI and in the population structure of methicillin-resistant Staphylococcus aureus belonging to clonal complex 8. Clin Microbiol Infect 2019 May;25(5):628.e1-628.e7.
- 13. Banerjee J, Madhavi K, Kiranmai S, Sureka RK. Screening for nasal carriage of methicillin resistant Staphylococcus aureus among healthcare workers at a Rural Teaching Hospital in Medchal District, Telangan. Int J Med Microbiol 2019;7(2):1-7.
- 14. Baruah K, Borthakur AK. Shamsuddin. Acteriological (aerobic) profile of chronic suppurative otitis media with antibiotic sensitivity testing in a tertiary care hospital of north east India. GJRA 2019;8(3):3.
- 15. Bhat YJ, Hassan I, Bashir S, Farhana A, Maroof P. Clinico-bacteriological profile of primary pyodermas in Kashmir: a hospital-based study. J R Coll Physicians Edinb 2016 Mar;46(1):8-13.
- 16. Bhatt MP, Bhalla GS, Tandel K, Jindamwar P, Chaudhari CN, Sahni NG. Antimicrobial susceptibility profile of methicillin-resistant Staphylococcus aureus at a tertiary care centre. Arch Clin Microbiol 2015;6(3):6.
- 17. Bhattacharya S, Bir R, Majumdar T. Evaluation of multidrug resistant Staphylococcus aureus and their association with biofilm production in a tertiary care hospital, Tripura, Northeast India. J Clin Diagn Res 2015 Sep;9(9):DC01-DC04.
- 18. Bhattacharyya C, Dey R, Roy T, Ghosh A, Jana H. Methicillin-resistant S. aureus in Eastern India: some molecular epidemiological perspectives. Int J Sci Stud 2017;4(11):66-69.
- 19. Bhavana J, Rama NK. Study of HA-MRSA and CA-MRSA isolated from clinical cases in a tertiary care hospital. Indian J Public Health Res Dev 2017;8(2):106-111.
- 20. Bhavana MV, Joshi S, Adhikary R, Beena HB. Mupirocin Resistance in Staphylococcus aureus in a tertiary care hospital of South India – a prospective study. Asian J Pharm Clin Res 2019;12(1):98-100.
- 21. Bhavsar R, Garala NJ, Garala RN, Patel P, Javadekar TB, Patel H, et al. Antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus isolated from various clinical samples at SSG hospital, Baroda. J Res Med Dent Sci 2015;3(1):43-46.
- 22. Bhowmik D, Chetri S, Paul D, Chanda DD, Bhattacharjee A. Detection and molecular typing of methicillin resistant Staphylococcus aureus from northeastern part of India. Med J Armed Forces India 2015;75(1):86-89.
- 23. Bhutia KO, Singh T, Adhikari L, Biswas S. Molecular characterization of community- & hospital-acquired methicillin-resistant & methicillin-sensitive Staphylococcus aureus isolates in Sikkim. Indian J Med Res 2015 Sep;142(3):330-335.
- 24. Bouchiat C, El-Zeenni N, Chakrakodi B, Nagaraj S, Arakere G, Etienne J. Epidemiology of Staphylococcus aureus in Bangalore, India: emergence of the ST217 clone and high rate of resistance to erythromycin and ciprofloxacin in the community. New Microbes New Infect 2015 May;7:15-20.
- 25. Chaudhary M, Payasi A. Surveillance study for MRSA prevalence and susceptibility trends against mecA and vanA positive clinical isolates. IJAPBC 2015;4(2):469-477.
- 26. Choudhury D, Chakravarty P. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus in Silchar Medical College and Hospital, Assam, India. Int J Basic Clin Pharmacol 2016;5(5):2174-2177.
- 27. Cugati S, Saikumar. Prevalence and antibiotic susceptibility pattern of Staphylococcus aureus isolated from blood culture in a teritiary care centre. J Med Sci Clin Res 2017;5(4):20842-20847.
- 28. Dass BS, Udayakumar P, Malaiyan J, Krishnaswamy B, Krishnan P. Prevalence of virulence determinants among HA-MRSA and CA-MRSA isolates and pathogenicity testing using Caenorhabdidtis elegans model. Int J Infect Dis 2016;45:76-77.
- 29. Datta P, Chander J, Gupta V, Mohi GK, Attri AK. Evaluation of various risk factors associated with multidrug-resistant organisms isolated from diabetic foot ulcer patients. J Lab Physicians 2019 Jan-Mar;11(1):58-62.
- 30. Deepika G, Anaparthy U, Krishna PBM. Prevalence of nasal carriage of MRSA among patients undergoing haemodialysis and in health care workers working in the haemodialysis units. J Pharm Sci Innov 2015;4(1):40-43.
- 31. Dhiman M, Bhatt N, Chau-Han V, Farooq U, Khan A. Emergence of methicillin resistant Staphylococcus aureus (MRSA) isolates from north India harboring a novel sasX gene: further analyzing its role in biofilm formation. Ann Med Biomed Sci 2017;3(1):46-50.
- 32. Dixit R. Carriage of Methicillin Resistant Staphylococcus aureus (MRSA) among health care workers in cardiac unit of a tertiary care hospital. Int.J.Curr.Microbiol. ASci 2018;7(3):2674-2679.
- 33. Farooq S, Saleem M. Prevalence of constitutive and inducible clindamycin resistance among clinical isolates of staph aureus in Kashmir valley: A hospital-based study. J Evol Med Dent Sci 2017;5(17):828-831.
- 34. Geetha SH, Kalghatgi AT, Rama NK. Characterization of methicillin resistant Staphylococcus aureus strains from clinical isolates in a tertiary care hospital of south India. Int J Med Res Rev 2015;3(9):1077-1083.
- 35. Ghosh S, Banerjee M. Methicillin resistance & inducible clindamycin resistance in Staphylococcus aureus. Indian J Med Res 2016 Mar;143(3):362-364.
- 36. Govindan S, Maroli AS, Ciraj AM, Bairy I. Molecular epidemiology of methicillin resistant staphylococcus aureus colonizing the anterior Nares of school children of Udupi Taluk. Indian J Med Microbiol 2015 Feb;33(1)(Suppl):129-133.
- 37. Gupta BP, Sinha S. Prevalence of methicillin resistant staphylococcus aureus in clinical samples of Teerthankar Mahaveer Medical College Hospital and Research Centre (TMMCH RC): Moradabad (UP): India. Int J Med Res Health Sci 2017;6(6):17-20.
- 38. Gupta L, Agarwal M, Bala K. Nasal carriage of methicillin resistant Staphylococcus aureus (MRSA) in health care workers and healthy individuals in a tertiary care hospital. Int J Pharm Biol Sci 2015;5(4):7-17.
- 39. Gupta S, Dongre A, Pandey AC, Biswas R, Gupta S. Antibiogram of methicillin resistant Staphylococcus aureus (MRSA) in healthcare settings. J Chem Pharm Res 2015;7(8):61-66.
- 40. Gupta M, Kaore N, Gupta A. Comparative evaluation of MIC by E-test and Cefoxitin disc diffusion for detection of Methicillin Resistant Staphylococcus aureus (MRSA). Indian J Microbiol Res 2016;3(4):407-410.
- 41. Gupta V, Pachori R, Goyal RK. Antibiotic susceptibility pattern of Staphylococcus aureus in tertiary care hospital, SRMSIMS, Bareilly, UP. Int J Com Med Pub Health 2017;4(8):2803-2809.
- 42. Hemamalini V, Kavitha V, Ramachandran S. In vitro antibiogram pattern of Staphylococcus aureus isolated from wound infection and molecular analysis of mecA gene and restriction sites in methicillin resistant Staphylococcus aureus. J Adv Pharm Technol Res 2015 Oct-Dec;6(4):170-175.
- 43. Hussain JH, Thakur A, Mishra B, Dogra V, Jaggi T. Antimicrobial susceptibility pattern of methicillin-resistant strains of Staphylococcus aureus in a super specialty hospital. Int J Health Allied Sci 2015;4(2):69-72.
- 44. Jana H, Roy T, Dey R, Dey JB, Ghosh A, Mondal KC. Mondal, Prevalence and antimicrobial susceptibility pattern of different clinical isolates of HA-MRSA and CA-MRSA in a tertiary care rural hospital, Bankura, West Bengal, India. Sch J App Med Sci 2015;3(2F):944-948.
- 45. Jindal N, Malhotra R, Grover P, Singh S, Bansal R, Kaur S. Methicillin resistant Staphylococcus aureus (MRSA) in Malwa region of Punjab (North-West India). Indian J Med Res 2016 Mar;143(3):371-372.
- 46. John SJ, Prakasan G, Sandeep PM, Sunil AE, Mukunda A, Sadiya RV. Nasal screening of dental students as MRSA carriers – An Institutional study. Indian J Appl Res 2019;9(6):30-32.
- 47. Joshi SA, Barua S, Swaminathan R. Prevalence of methicillin resistant Staphylococcus aureus in a tertiary care hospital in Navi Mumbai, India. Indian J Microbiol Res 2017;4(2):187-189.
- 48. Kaur K, Gill AK, Kaur M. Methicillin resistance, vancomycin intermediate and vancomycin resistance Staphylococcus aureus prevalence in a tertiary care hospital of Punjab, India. National J Lab Med 2019;8(3):MO01-MO03.
- 49. Kavitha Y, Mohan S, Moinuddin SK. Bacteriological profile of diabetic foot infection with special reference to ESBL and MRSA in a coastal tertiary care teaching hospital. Indian J Microbiol Res 2017;4(1):68-73.
- 50. Kogekar SP, Jain K, Kumari P, Chavan N, Peshattiwar P, Rajput MS. High level of MRSA colonization in health care worker: alarm to implement health care policy. World J Clin Pharmacol Microbiol Toxicol 2015;1(2):21-25.
- 51. Kulshrestha A, Anamika V, Mrithunjay K, Himanshu V, Manish K, Dalal AS. A prospective study on the prevalence and antibiotic sensitivity pattern of methicillin resistant Staphylococcus aureus isolated from various clinical specimen at a tertiary care post graduate teaching institute. Int J Curr Microbiol Appl Sci 2017;6(3):1859-1869.
- 52. Kulshrestha M, Ghatak T, Gupta P, Singh M, Agarwal J. Surveillance of health-care workers for nasal carriage to detect multidrug-resistant Staphylococcus s in a tertiary care center: an observational study. Med J DY Patil Vidyapeeth 2019;12(11):39-43.
- 53. Kumar S, Bhadauria S. Increasing trend of methicillin-resistant Staphylococcus aureus in Jaipur, Rajasthan, India. Am J Ment Retard 2016;10(34):1417-1421.
- 54. Kumari J, Shenoy SM, Baliga S, Chakrapani M, Bhat GK. Healthcare-associated methicillin-resistant Staphylococcus aureus: clinical characteristics and antibiotic resistance profile with emphasis on macrolide-lincosamide-streptogramin B resistance. Sultan Qaboos Univ Med J 2016 May;16(2):e175-e181.
- 55. Majhi S, Dash M, Mohapatra D, Mohapatra A, Chayani N. Detection of inducible and constitutive clindamycin resistance among Staphylococcus aureus isolates in a tertiary care hospital, Eastern India. Avicenna J Med 2016 Jul-Sep;6(3):75-80.
- 56. Mamtora D, Saseedharan S, Bhalekar P, Katakdhond S. Microbiological profile and antibiotic susceptibility pattern of Gram-positive isolates at a tertiary care hospital. J Lab Physicians 2019 Apr-Jun;11(2):144-148.
- 57. Mehta S. Incidence of methicillin resistant Staphylococcus aureus and its antibiotic susceptibility pattern in a tertiary care hospital. Indian J Microbiol Res 2017;4(1):40-43.
- 58. Mendem SK, Triveni AG, Shivannavar CT, Gaddad SM. Prevalence of MRSA and VRSA in Kalaburagi region. Int J Pharm Biol Sci 2016;6(3):81-85.
- 59. Mohanty S, Behera B, Sahu S, Praharaj AK. Recent pattern of antibiotic resistance in Staphylococcus aureus clinical isolates in Eastern India and the emergence of reduced susceptibility to vancomycin. J Lab Physicians 2019 Oct-Dec;11(4):340-345.
- 60. Mokta KK, Verma S, Chauhan D, Ganju SA, Singh D, Kanga A, et al. Inducible clindamycin resistance among clinical isolates of Staphylococcus aureus from Sub Himalayan Region of India. J Clin Diagn Res 2015 Aug;9(8):DC20-DC23.
- 61. Mondal H, Gupta I, Nandi P, Ghosh P, Chattopadhyay S, Mitra GD. Nasal screening of healthcare workers for nasal carriage of methicillin resistance Staphylococcus aureus, vancomycin vancomycin resistance Staphylococcus aureus and prevalence of nasal colonization with Staphylococcus aureus in Burdwan Medical College and Hospital. Med Coll Hosp 2016;3(11):3342-3346.
- 62. Mundhada SG, Shaikh NK, Gudlani VR, Bhise MP, Ingole KV. Screening for methicillin-resistant Staphylococcus aureus (MRSA) carriage among the health care workers in a tertiary care center. Indian J Microbiol Res 2017;4(2):150-153.
- 63. Mushtaq A, Imran S, Bhat MY, Hilal N. Prevalence and antimicrobial susceptibility of methicillin resistant staphylococcus in a tertiary Care hospital in Kashmir. Int J Adv Res (Indore) 2016;4(4):267-271.
- 64. Nadimpalli G, Bhamare S, Rao NP, Ingole S. Incidence of methicillin-resistant Staphylococcus aureus (MRSA) infection among patients and hospital staff and impact of preventive measures in reduction of MRSA infection rate: a prospective observational stud. Int J Basic Clin Pharmacol 2016;5(6):2336-2340.
- 65. Nagamadhavi V, Samatha P. Assessing the prevalence of Staphylococcus Aureus, Particularly MRSA, from anterior nares of medical students. Indian J Microbiol Res 2016;3(1):22-23.
- 66. Nagaraju U, Raju BP. Methicillin-resistant Staphylococcus aureus in community-acquired pyoderma in children in South India. Indian J Paediatr Dermatol 2017;18(1):14-17.
- 67. Nagasundaram N, Sistla S. Existence of multiple SCCmec elements in clinical isolates of methicillin-resistant Staphylococcus aureus. J Med Microbiol 2019 May;68(5):720-727.
- 68. Negi V, Pal S, Juyal D, Sharma MK, Sharma N. Bacteriological profile of surgical site infections and their antibiogram: a study from resource constrained rural setting of Uttarakhand State, India. J Clin Diagn Res 2015 Oct;9(10):DC17-DC20.
- 69. Pai AK, Kamath MR, Karnaker VK. Nasal colonisation with MRSA in patients undergoing cardiac surgery. Int J Biol Med Res 2015;6(3):5131-5134.
- 70. Pai AK, Kamath MR, Karnaker VK, Rai R, Gopalakrishnan M. Mupirocin and vancomycin susceptibility in MRSA colonising anterior nares of patients scheduled for cardiac surgery. Indian J Microbiol Res 2017;4(1):83-86.
- 71. Pal S, Sayana A, Joshi A, Juyal D. Staphylococcus aureus: A predominant cause of surgical site infections in a rural healthcare setup of Uttarakhand. J Family Med Prim Care 2019 Nov;8(11):3600-3606.
- 72. Pandya N, Chaudhary A, Mehta S, Parmar R. Characterization of methicillin resistant Staphylococcus aureus from various clinical samples at tertiary care hospital of rural Gujarat. Int J Health Sci Res 2015;5(9):202-206.
- 73. Patil AB, Chikkaraddi U, Sambrani PN, NR S, KR SM. Trends in antibiotic resistance among methicillin-resistant Staphylococcus aureus (MRSA) isolate. Indian J Microbiol Res 2017;4(4):454-458.
- 74. Patil AK, Namineni S, Cheruku SR, Penmetsa C, Penmetcha S, Mallineni SK. Prevalence of community-associated methicillin-resistant Staphylococcus aureus in oral and nasal cavities of 4 to 13-year-old rural school children: a cross-sectional study. Contemp Clin Dent 2019 Jan-Mar;10(1):99-104.
- 75. Perala BM, Koripella RL, Vani TM. Prevalence of methicillin resistant Staphylococcus aureus in a tertiary care hospital. IOSR J Dent Med Sci 2016;15(5):29-31.
- 76. Perween N, Krishanprakash S, Bharara T. Bacteriological profile of burn wound infection in a tertiary care hospital in north India with special reference to methicillin resistant Staphylococcus aureus. IMJ Health 2015;8:6-11.
- 77. Phukan C, Ahmed GU, Sarma PP. Inducible clindamycin resistance among staphylococcus aureus isolates in a tertiary care hospital of Assam. Indian J Med Microbiol 2015 Jul-Sep;33(3):456-458.
- 78. Radhakrishna M, Taneja A, Rao P. Nasal carriage of Staphylococcus aureus with special emphasis on methicillin-resistant Staphylococcus aureus among students of a south Indian Medical College — Prevalence and antibiogram pattern. Asian J Pharm Clin Res 2016;9(2):129-132.
- 79. Raigar M, Vyas A, Sharma R, Malhotra B. Detection of vancomycin intermediate and vancomycin resistance in clinical isolates of Staphylococcus aureus in Tertiary Care Hospital. IJMHR 2019;5(6):01-04.
- 80. Khara R, Lakhani SJ, Vasava S, Shah K, Panjwani D. Methicillin resistant Staphylococcus aureus (MRSA) and vancomycin resistant Staphylococcus aureus (VRSA) from a rural based tertiary care and teaching hospital in Vadodara district, Gujarat. IAIM 2016;3(7):187-195.
- 81. Reema HA, Dominic SR. Prevalence and antimicrobial susceptibility pattern of clinical isolates of methicillin-resistant Staphylococcus aureus in a tertiary care hospital in Mangalore. J Int Med Dent 2016;3(3):134-139.
- 82. Rengaraj R, Mariappan S, Sekar U, Kamalanadhan A. Detection of vancomycin resistance among enterococcus faecalis and Staphylococcus aureus. J Clin Diagn Res 2016 Feb;10(2):DC04-DC06.
- 83. Routray S, Rath S, Mohanty N. Prevalence of methicillin resistant Staphylococcus aureus isolated from saliva samples of patients with oral squamous cell carcinoma. J Oral Res 2019;8(1):30-36.
- 84. Roy SS. Spectrum of Public Health Significant and Antibiotic Resistant Microorganisms Isolated from Mobile Phones of Meat and Fish Handlers of Tripura, India. Int. J. Curr. Microbiol Sci 2018;7(7):13-204.
- 85. Rudresh MS, Ravi GS, Motagi A, Alex AM, Sandhya P, Navaneeth BV. Prevalence of Mupirocin resistance among Staphylococci, its clinical significance and relationship to clinical use. J Lab Physicians 2015 Jul-Dec;7(2):103-107.
- 86. Sankaran G, Zacharia B, Roy A, Purayil SP. Current clinical and bacteriological profile of septic arthritis in young infants: a prospective study from a tertiary referral centre. Eur J Orthop Surg Traumatol 2018 May;28(4):573-578.
- 87. Selvabai AP, Sattar SB, Jayaraman P, Shanmugam P. Detection and characterisation of heteroresistant vancomycin intermediate Staphylococcus aureus (hVISA) using phenotypic and genotypic methods. J Clin Diagn Res 2019;13(5):DC01-DC05 .
- 88. Sengupta M, Banerjee S, Banerjee P, Guchhait P. Outstanding prevalence of methicillin resistant Staphylococcus aureus in neonatal omphalitis. J Clin Diagn Res 2016 Sep;10(9):DM01-DM03.
- 89. Senthilkumar K, Biswal N, Sistla S. Risk factors associated with methicillin-resistant Staphylococcus aureus infection in children. Indian Pediatr 2015 Jan;52(1):31-33.
- 90. Shinde RV, Pawar SK, Mohite RV, Shinde AR, Duggu P. Study of nasal carriage of Staphylococcus aureus with special reference to methicillin resistance among nursing staff. Arch Clin Microbiol 2017;7:1-6.
- 91. Singh S, Malhotra R, Grover P, Bansal R, Galhotra S, Kaur R, et al. Antimicrobial resistance profile of Methicillin-resistant Staphylococcus aureus colonizing the anterior nares of health-care workers and outpatients attending the remotely located tertiary care hospital of North India. J Lab Physicians 2017 Oct-Dec;9(4):317-321.
- 92. Singh G, Broor S, Agarwal P. Molecular characterisation of Staphylococcus aureus using spa typing as a diagnostic tool in Haryana, India. Indian J Med Microbiol 2018 Jan-Mar;36(1):26-31.
- 93. Singh N, Mohanty S, Panda SS, Sahoo S, Pattnaik D, Jena J. Methicillin resistant Staphylococcus aureus (MRSA) carriage among health care workers in a tertiary care hospital in Bhubaneswar. Int J Community Med Public Health 2018;5(8):3276-3282 .
- 94. Chinnambedu RS, Marimuthu RR, Sunil SS, Amrose P, Ramachandran V, Pachamuthu B. Changing antibiotic resistance profile of Staphylococcus aureus isolated from HIV patients (2012-2017) in Southern India. J Infect Public Health 2020 Jan;13(1):75-79.
- 95. Talwar A, Saxena S, Kumar A. Screening for detection of methicillin-resistant Staphylococcus aureus in Doon Valley Hospitals, Uttarakhand. J Environ Biol 2016 Mar;37(2):247-251.
- 96. There YW, Wadhai VS, Bhandari P. Prevalence of vancomycin resistance Staphylococcus aureus among MRSA isolates from district hospital Gadchiroli (m.s.) India. IJRBAT 2018;4(1):214-218.
- 97. Thomas MG, Nair SP. MRSA in dermatology inpatients with a vesiculobullous disorder. Cutis 2018 Jun;101(6):458-461.
- 98. Tiewsoh JB, Dias M. Screening of methicillin-resistant Staphylococcus aureus in healthcare workers and students and its susceptibility to mupirocin in a tertiary care teaching hospital in South India. J Lab Physicians 2017 Oct-Dec;9(4):239-242.
- 99. Tripathi A. Prevalence and antimicrobia pattern of methicillin resistant Staphylococcus aureus in central India. Med Pulse Int Med J 2015;2(1):45-48.
- 100. Trivedi MB, Vegad M, Soni S. Prevalence of methicillin-resistant Staphylococcus aureus in various clinical samples in a tertiary-care hospital. Int J Med Sci Public Health 2015;4(12):1735-1738 .
- 101. Vasuki V, Ananthasankari S. Evaluation of antibiotic resistance among methicillin resistant Staphylococcus aureus isolates in a tertiary care teaching hospital, South India. IJCMAAS 2016;10(2):46-49.
- 102. Velayudham A, Manavalan J, Vadivel S, Kaliyamoorthy B, Kuthalaramalingam S. Bacteriophage typing of methicillin resistant Staphylococcus aureus and changing trend in their antibiotic profile. Ann Int Med Den Res 2017;3(1):MB01-MB05.
- 103. Venkatesan P, Shanmugam P, Satter SB. Bacteriological profile and resistance pattern of the microorganisms causing bacteraemia with special emphasis on unusual blood isolates. Indian J Microbiol Res 2017;4(2):171-176.
- 104. Arabestani MR, Rastiyani S, Alikhani MY, Mousavi SF. The relationship between prevalence of antibiotics resistance and virulence factors genes of MRSA and MSSA strains isolated from clinical samples, West Iran. Oman Med J 2018 Mar;33(2):134-140.
- 105. Goudarzi M, Fazeli M, Eslami G, Pouriran R, Hajikhani B, Dadashi M. Genetic diversity analysis of methicillin-resistant Staphylococcus aureus strains isolated from intensive care unit in Iran. Oman Med J 2019 Mar;34(2):118-125.
- 106. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8(5):336-341.
- 107. Greco T, Zangrillo A, Biondi-Zoccai G, Landoni G. Meta-analysis: pitfalls and hints. Heart Lung Vessel 2013;5(4):219-225.