|
Abstract
A 32 -year- old male presented with complaints of fever, dry cough, breathlessness and right sided chest pain of two months duration. Chest radiograph showed right sided hydropneumothorax which revealed frank pus on diagnostic thoracocentesis, for which tube thoracostomy was done. Despite vigorous broad spectrum antibiotic coverage, postural drainage and chest physiotherapy, there was no clinical improvement. Further work up included serology, pleural fluid culture, closed as well as thoracoscopic guided pleural biopsy revealed growth of Aspergillus fumigatus. Patient was prescribed antifungal medication (Voriconazole) and subsequent thoracotomy with right sided pneumonectomy showed good clinical recovery.
Keywords: Aspergillosis; Aspergillus fumigatus; Aspergillus pyopneumothorax.
Introduction
Aspergillus is an ubiquitous saprophytic mould that has been found in soil, in decaying organic matter and in bedding.1 The infection may manifest with clinically and radiologically distinct patterns including saprophytic aspergillosis (aspergilloma), allergic bronchopulmonary aspergillosis, semi-invasive aspergillosis, airway invasive aspergillosis and angioinvasive aspergillosis.2 Fungal disease accounts for less than 1% of all pleural effusions.3 The most common cause is aspergillus infection (usually Aspergillus fumigatus). As Aspergillus pyopneumothorax is a rare clinical entity; to our knowledge, the present case represents the first ever reported from India.
Case Report
A 32-year-old male patient with history of antituberculosis treatment as therapeutic trial for the last one month was admitted at the department of pulmonary medicine, with complaints of low grade fever, dry cough, right sided chest pain and progressive breathlessness of 2 months duration. He had no history of hemoptysis and loss of appetite. Past history revealed no cardiac illness, bronchial asthma, hypertension, diabetes mellitus, any previous hospitalization or surgical intervention. His family history was also unremarkable. There was no history suggestive of congenital or acquired immunosuppression. On general examination, the patient was toxic with a pulse rate of 116/min, respiratory rate 32/min and blood pressure 110/76 mmHg. Respiratory examination revealed asymmetrical chest with tracheal shift towards the left, diminished movement and vocal fremitus on the right side with dull note in the mammary, infraaxillary and infrascapular region. Hyperresonant note was observed in the axillary and right interscapular region with positive succussion splash and shifting dullness. Breath sounds and vocal resonance were diminished on corresponding areas of the right side with no adventitious sounds. The rest of physical examination was unremarkable.
He had normal hematological and biochemical parameters. Serology for HIV was negative. ECG was within normal limits. Arterial blood gas analysis showed type I respiratory failure (PaO2: 48mmol/l; PaCO2: 35.6mmol/l; pH: 7.38; HCO3: 22.8meq/l; SaO2=86%). Mantoux test was negative. Chest radiograph showed right sided hydropneumothorax without shift of medistinum towards opposite side (Fig. 1). Diagnostic thoracocentesis revealed frank thick pus and tube thoracostomy was done. Subsequent radiograph showed persistence of air fluid level (Fig. 2). Pleural fluid analysis revealed; protein (4.7gm/dl), sugar (128 mg/dl), albumin (0.22 gm/dl), lactate dehydrogenase (1819 IU/L) and total leucocyte count (6800/cm3) with neutrophilic predominance (78%). Acid fast bacilli and Gram’s staining were negative.
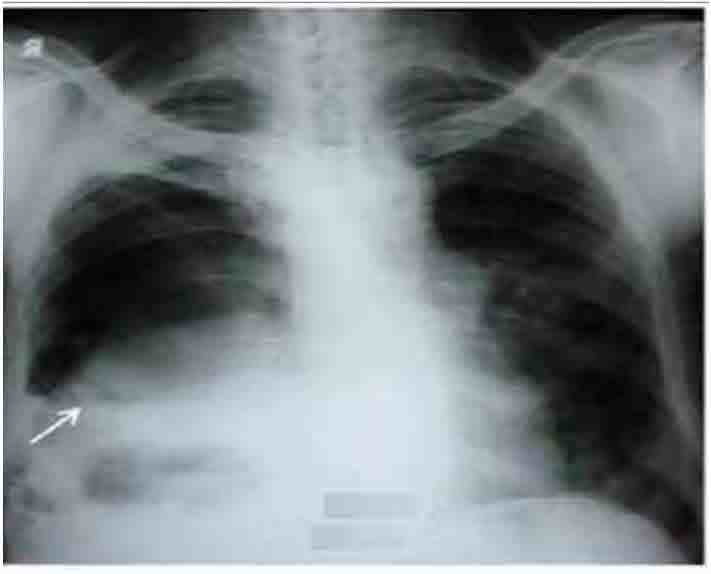
Figure 1: Chest radiograph showing right sided hydropneumothorax without shift of mediastinum towards opposite side (arrow).
The patient was advised to continue antituberculosis therapy (rifampicin, isoniazid, ethambutol and pyrazinamide) previously prescribed along with broad spectrum intravenous antibiotics (cefoperozone + sulbactam; amikacin and metronidazole). Thereafter, sputum and pus were studied for acid fast bacilli and Gram’s staining and culture and sensitivity for Mycobacterium tuberculosis and pyogenic organisms. All were found to be negative. Despite antibiotic coverage, the patient was toxic and tachypneic with no reduction in intercostal tube drainage and without improvement in serial repeat chest skiagrams.
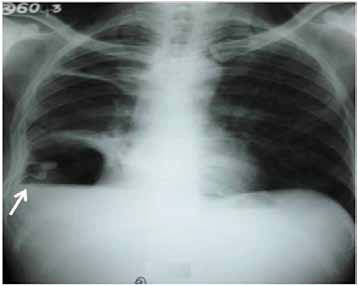
Figure 2: Chest radiograph showing persistence of air fluid level after tube thoracostomy (arrow).
Further work up included ultrasonography of the thorax that showed evidence of loculated right-sided moderate pleural collection with internal air echoes. Computed tomography of the thorax revealed right sided pyopneumothorax with collapse consolidation of underlying lung parenchyma (Fig. 3). As pleural fluid was brown and turbid in nature, a suspicion of fungal etiology was made and the same was sent for fungal smear as well as culture. The smear showed plenty of septate hyphae with conidiophore suggestive of aspergillus infection. Subsequently, pleural fluid culture showed Aspergillus fumigatus. Skin prick testing showed hypersensitivity to Aspergillus fumigatus. The total serum IgE and IgE specific to Aspergillus fumigatus were elevated (1490.2 IU/ml and 4.8 IU/ml, respectively). Diagnostic thoracoscopy revealed multiple fibrinous adhesions and loculations along with fibrinous exudates lining the pleural and diaphragmatic surfaces. Closed pleural biopsy by Cope needle was also performed along with thoracoscope guided pleural biopsy. Histological evaluation showed an acute inflammatory infiltration with necrosis and vasculitis (Fig. 4), Gomori’s methenamine silver stain showed branching slender fungal hyphae (Fig. 5). Culture showed growth of Aspergillus fumigatus.
The patient was started on antifungal treatment (Intravenous voriconazole, 400 mg twice daily for the first day, followed by 200 mg twice daily) after withholding previous medications and was posted for surgical intervention.
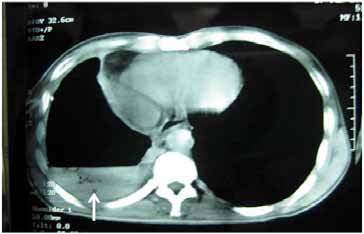
Figure 3: Computed tomography of thorax showing right sided air fluid level with collapse consolidation of underlying lung parenchyma (arrow).
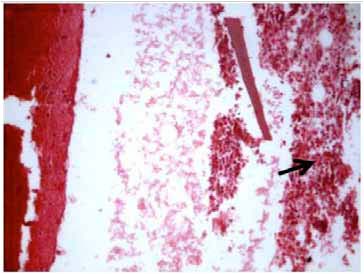
Figure 4: Microphotograph of the pleural biopsy specimen showing acute inflammatory infiltrate (arrow) with fibrosis and necrosis (Hematoxylin and Eosin × 125 × digital magnification).
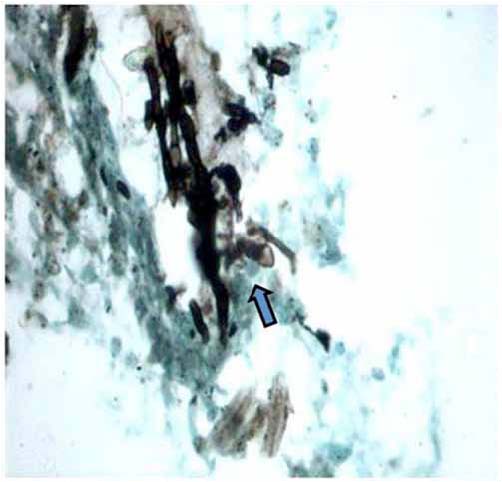
Figure 5: Microphotograph of pleural biopsy showing slender fungal branching hyphae (arrow) invading vessel wall (Gomori methanamine silver stain × 200 × digital magnification).
Thoracotomy with right sided pneumonectomy was done without having any post-operative complication and systemic antifungal treatment was continued further. He was discharged on oral voriconazole 200 mg twice daily for six months. The patient was followed up for nine months and showed favorable recovery.
Discussion
Aspergillosis is the collective term used for describing all disease entities caused by various species of Aspergillus such as A. fumigatus, A flavus, A. niger, A. terreus and others. Among all species, Aspergillus fumigatus is responsible for a large spectrum of diseases. Airway colonization by aspergillus is usually seen in patients with chronic lung lesions, such as bronchiectatic cavities, pulmonary fibrosis, or tuberculosis sequelae, and in conditions were local host defense is impaired. Among all the conditions tuberculosis remains the major risk factor for developing aspergillosis. Aspergillus of the pleura is rare and can occur following lobectomy or pneumonectomy for tuberculosis or lung cancer with bronchopleural fistula or in patients treated in the past with pneumothorax therapy for tuberculosis.4 Aspergillosis usually develops in immunocompromised patients but it can can develop in immunocompetent as well.5-7 Aspergillus empyema thoracis is very uncommon and caused by rupture of an aspergilloma cavity or as a complication of a pre-existing chronic empyema.8 Cases of pyopneumothorax secondary to aspergillus infection have been reported rarely.9-11 In the current case, infection occurred primarily without having any pre-existing pulmonary sequelae and long treatment history. The patient was apparently healthy without any history of immunosupression. It was difficult to determine radiologically whether aspergillus infection occurred primarily as result of pleural disease or after rupture of a lung cavity into pleura, but the former seems to occur more likely as no lung cavity was observed in chest radiograph as well as computed tomography of the thorax. However, the diagnosis of Aspergillus fumigatus was established on the basis of tissue biopsy as well as pleural fluid culture.
The optimal treatment for pleural aspergillosis is early excision of involved pleura with resection of the upper lobe or in severe cases, the entire ipsilateral lung.12 Before the definitive surgical intervention, anti-fungals should be administered systemically before and after the operation because the incidence of post operative infection with Aspergillus is high. Since antifungal drugs like amphoterecin B have been used both parenterally as well as in the form of intrapleural infusion over decades, newer antifungals like itraconazole, voriconazole as well as posaconazole are also effective.13 In our patient voriconazole was preferred because it is considered to be a potentially safe and effective first-line treatment for invasive aspergillosis. It also has better bioavailability, overall response rate and minimum probability of resistance to Aspergillus fumigatus as compared to other anti-fungal agents.13 The patient showed good recovery mainly because of early diagnosis, prompt surgical intervention and good antifungal coverage.
Conclusion
The incidence of fungal empyema thoracis as well as pyopneumothorax has been increased in recent years. Suspicion of fungal etiology should be made on the basis of no response despite broad-spectrum antibiotic coverage, and evidence of pre-existing immunosuppression. Early diagnosis, appropriate surgical interventions as well as antifungal therapy improve the patient outcome despite high mortality.
Acknowledgements
The author reported no conflict of interest and no funding was received in this work.
References
1. Buckingham SJ, Hansell DM: Aspergillus in the lung: diverse and coincident forms. Eur Radiol 2003; 13:1786-1800.
2. Franquet T, Müller NL, Giménez A, Guembe P, de La Torre J, Bagué S.Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics 2001; 21:825-37.
3. Light RW: Pleural effusion secondary to fungal infections, actinomycosis, and nocardiosis: Pleural disease. 4th ed. Baltimore: Williams and Wilkins 2001; 11:196-203.
4. Wex P, Utta E, Drozdz W: Surgical treatment of pulmonary and pleura- pulmonary Aspergillus disease. Thorac Cardiovasc. Surg. 1993; 41:64-70.
5. Ko JP, Kim DH, Shepard JA. Pulmonary aspergillosis in an immunocompetent patient. J Thorac Imaging 2002; 17:70-73.
6. Kaya S, Yavuz I, Cobanoglu U, Ural A,Yilmaz G, Koksal I. Fatal sino-orbital aspergillosis in an immunocompetent case. Mikrobiyol Bul 2011; 45:546-552.
7. Kose S, Cavdar G, Senger SS, Akkoclu G. Central nervous system aspergillosis in an immunocompetent patient. J Infect Dev Ctries 2011; 5:313-315.
8. Ko SC, Chen KY, Hsueh PR, Luh KT, Yang PC: Fungal empyema thoracis: an emerging clinical entity. Chest 2000; 117:1672-1678.
9. Van Klink HC, Stolk J. Pneumothorax as a complication of chronic necrotizing pulmonary aspergillosis. Ned Tijdschr Geneeskd 2005; 149:598-600.
10. Zhang W, Hu Y, Chen L, Gao J, Xie L. Pleural aspergillosis complicated by recurrent pneumothorax: a case report. Journal of Medical Case Reports 2010; 4:180.
11. Denning DW, Kostantinos R, Dobrashian R, Sambatakou H. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: Case series, proposed nomenclature change and review. Clin Infect Dis 2003;37:s265-280
12. Hillerdal G .Pulmonary Aspergillus infection invading the pleura. Thorax1981; 36:745-751.
13. Walsh TJ, Anaissie EJ, Denning WD, Herbrecht R, Kontoyiannis DP, Marr KA et al. Treatment of Aspergillosis : Clinical practice guidelines of Infectious Diseases Society of America. Clin Infect Dis 2008; 46; s327-360.
|