In December 2019, a novel coronavirus was identified to cause pneumonia in a large number of people in the city of Wuhan in China.1 The virus spread rapidly leading to a global pandemic.1 The World Health Organization (WHO) designated the SARS-CoV-2 infection as COVID-19.1 COVID-19 infection is associated with high short-term morbidity, and in very severe cases, mortality. Post COVID-19 complications may lead to a major long-term impact on communities and health care systems. This is because cases of pulmonary sequelae are expected to rise significantly due to the very large number of former COVID-19 patients.2,3 The clinical and radiological features of acute SARS-CoV-2 infection have been well described in multiple studies. However, its long-term consequences on the lungs are not yet well described. Most emergent literature are limited to case reports, case series, or observational studies. This review article summarizes the known long-term respiratory complications of COVID-19 based on the current available evidence.
Symptomatology
It has been noticed that up to 75% of patients failed to regain normal health after recovering from COVID-19, fatigue being the main complaint, followed by muscle weakness, anxiety, depression, and sleep disturbances.2,3 These post-COVID-19 symptoms have been observed irrespective of the radiological and physiological findings.4 In a cross-sectional study by Galal et al,5 on 430 recovered COVID-19 patients in Egypt, 86% were found to have post-COVID-19 symptoms. Myalgia and arthralgia were present in 60% while 30% had chest pain and dyspnea. Such symptoms were more prevalent among patients with comorbidities and those who had severe acute phase of COVID-19.5 Dyspnea is one of the commonest symptoms that persist after COVID-19, with various studies reporting between 23% and 66% patients suffering from significant residual shortness of breath after 8–12 weeks of discharge, with a few requiring supplemental oxygen.6,7 These patients were experiencing the symptoms of what is informally known as the ‘Long COVID’. It has been observed more in females aged < 50 years for unclear reasons, perhaps because women are more likely to survive the severe acute phase than men.8-10 [Table 1].
Nalbandian et al,6 have suggested the term chronic post-COVID-19 syndrome to be used as a collective term for the symptoms, clinical and physiological features that persist beyond 12 weeks from the onset of acute COVID-19 without other clear causes.
Radiology
High resolution computed tomography (HRCT) plays an important role in diagnosing acute COVID-19 cases and monitoring the disease process during hospitalization.11 Monitoring radiological changes post viral pneumonia is also important.12 A recent systematic review and meta-analysis in patients with post non-COVID-19 viral pneumonitis reported ground glass opacity (GGO), consolidation, and fibrosis.12 Although GGO and consolidation improved over time, fibrosis persisted for years. In a prospective study from Beijing that investigated the long-term effect of SARS infection among health care workers reported that after 12 months from recovery, the damage indicated by CT scan had reduced to < 10% of the lung.13 However, the same study’s 15-year follow-up of SARS survivors showed that most parenchymal recovery was restricted to the first year and remained stable thereafter.13 In a single-center prospective study investigating the radiological changes of SARS infection six weeks post discharge from hospital, the major interstitial changes were organizing pneumonia (OP) in 59% and GGO in 38%.14 The GGO and irregular lines observed in post-COVID-19 pneumonia are similar to the long-term changes seen after SARS and influenza.2 The radiological features of post COVID-19 pneumonia are variable. These range from GGO with or without consolidation, interstitial thickening, parenchymal bands, and crazy paving to traction bronchiectasis.15 These changes are mainly peripherally distributed. Less common features include bronchiectasis, lymphadenopathy, and pleural effusion.15
Table 1: Summary of post-COVID-19 features.
GGO: ground glass opacity; FVC: Forced vital capacity; FEV1: Forced expiratory volume in the first second; TLC: Total lung capacity; DLCO: Diffusion capacity of carbon monoxide; 6MWT: Six-minute walk test;
VO2 max: Maximum oxygen consumption.
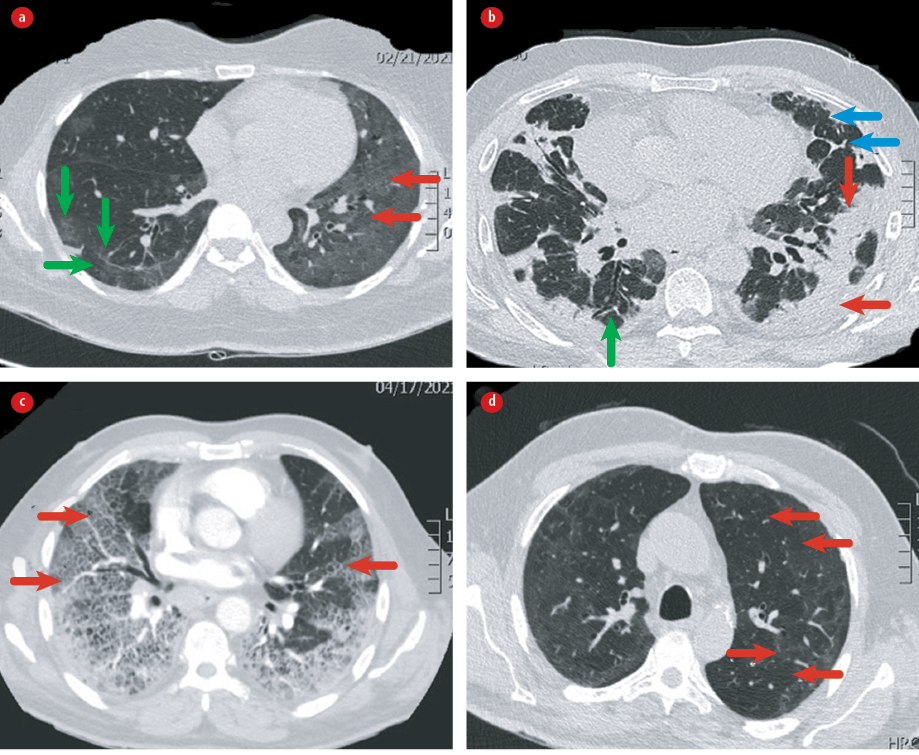
Figure 1: HRCT showing different patterns: (a) diffuse ground glass opacity (red arrows) and parenchymal bands (green arrows); (b) COP (red arrows), reticulations (blue arrows), and traction bronchiectasis (green arrow); (c) crazy paving; and (d) Mosaic attenuation.
Mosaic attenuation is another common finding post COVID-19 which may indicate either small airway disease or pulmonary vascular disease (PVD) [Table 1 and Figure 1].16 Although some HRCT features such as GGO and consolidation resolve with time, others like reticulation and parenchymal bands persist.15 In addition, in a study among patients who had severe COVID-19 pneumonia, 44% had abnormal chest X-ray (CXR) at six months.17 In one cohort followed up for 4–6 weeks post-COVID-19, 32% had interstitial changes on the CT chest.4 Another study showed that six months after recovery from severe COVID-19 pneumonia, 35% had fibrotic like changes, 27% had residual GGO, and 38% had complete radiological resolution.18 In the same study, age > 50 years and severe disease during the acute phase were predictors of fibrotic changes, which were found significantly decreased on follow-up CT at six months. A systematic review and meta-analysis of 15 studies investigating (in over 3000 patients) follow-up CT scans and pulmonary function tests (PFT), post COVID-19 at 1 to 6 months after discharge (average three months) showed residual CT changes in 55.7%.19 It is not clear yet whether the post-COVID-19 fibrotic changes are likely to persist or regress with time. We recommend a series of chest CT studies over one year and later that examine the relationship between patterns longitudinally.20
Table 2: Risk factors for post-COVID-19 pulmonary fibrosis.
|
|
- High inflammatory markers including CRP, IL-6, serum LDH and D-dimer levels.
|
- Length of hospitalization > 20 days.
|
- Length of ICU stay and mechanical ventilation.
|
- Initial CT findings (coarse reticular pattern, parenchymal bands, irregular interface, and interstitial thickening).
|
|
|
CRP: C-reactive protein; IL-6: Interleukin 6; LDH: Lactate dehydrogenase; ICU: Intensive care unit; CT: Computed tomography.
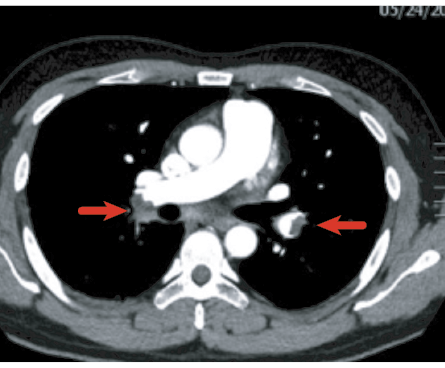
Figure 2: Bilateral filling defects in the main pulmonary arteries (arrows) representing bilateral pulmonary embolism.
Physiology
Impaired respiratory function is one of the sequelae of COVID-19 pneumonia. PFT showed that total lung capacity (TLC), forced vital capacity (FVC), and forced expiratory volume were lower than the normal reference values after severe COVID-19.16 Lower results in diffusing capacity for carbon monoxide (DLCO), a sensitive test, is indicative of the most common post-COVID-19 pulmonary complications.17 DLCO also has the advantage of being able to assess both the interstitial and the vascular components.17,21 It's results also correlate with disease severity.16 However, different studies give different rates of prevalence of impaired DLCO due to variations in the time point of post-recovery testing, heterogeneity of the patients, different selection criteria, research objectives, etc. For example, consider two different studies conducted on DLCO levels in COVID-19 recovered patients all of whom had severe pneumonia during the acute phase. In the first study, a systematic review and meta-analysis comprising a total of 380 cases from seven studies from China and France, > 60% patients were found with impaired DLCO.22 In the second study, on 114 patients in China conducted six months after recovery, 26% had DLCO levels
< 80% of the predicted levels (normal 80%–120%).18 The dearth of comparable studies may be due to the novelty of the post-COVID-19 phenomenon.
A prospective, multicenter follow-up study found that post-COVID-19 patients who required only oxygen or invasive mechanical ventilation (IMV) during acute phase were more likely to have severe DLCO impairment compared to those who required non-invasive ventilation (NIV) and without major differences in the other physiological parameters.17 This was because patients who required only oxygen were less likely to receive steroids and heparin compared to the sicker group. This study was conducted before the results of the recently launched post-COVID-19 initiative, the RECOVERY Trial, came out.23 Mo et al,24 who performed spirometry and DLCO in 110 patients with COVID-19 on the day of discharge from hospital, were able to show that 47.2% had impaired DLCO and 25% had reduced TLC, and that these were related to the severity of the disease. In a recent systematic review of studies conducted 12 months on former COVID-19 patients who had acute viral pneumonitis, approximately 40% had abnormal DLCO and about 25% had reduced TLC.22 In cohorts of patients who survived COVID-19 severe pneumonia, DLCO impairment was reported in 58% at 6 months.12,17,25 Another study reported that VO2 max declined in patients post COVID-19.26 The respiratory obstructive pattern has also been reported in patients who recovered from COVID-19 pneumonia.22 In a Chinese study, about 25% of post-COVID-19 patients after 6 months, the median 6-minute walking distance was found lower than the reference values for comparable healthy people, and was similar to what was reported in SARS and MERS-CoV survivors2,27 [Table 1].
On the other hand, a recent study suggested that early physiological assessment upon discharge could overestimate the long-term impact of COVID-19 as many factors contribute to impairments in the acute phase.28 Consequently, the current British Thoracic Society (BTS) guidelines recommend performing PFT for COVID-19 patients at three months’
post discharge.29
Pulmonary fibrosis
Viral pneumonia is a potential cause of pulmonary fibrosis. Two retrospective observational longitudinal studies demonstrated that viral pneumonia raised the risk for pulmonary fibrosis by 20%.30 In addition, the patients who had viral pneumonia that led to pulmonary fibrosis were relatively younger. As per the systematic review and study by Fabbri et al,12 the fibrotic changes post-viral infection (SARS-CoV-2 and influenza) can persist for years. Pulmonary fibrosis is estimated to persist in one-third of the survivors of COVID-19 who had severe pneumonia.25 Post-COVID-19 fibrosis at four months had a much higher incidence among those who had been mechanically ventilated (72%) during the acute phase than among the non-ventilated patients (20%).31 Moreover, most patients who develop fibrosis are male, elderly, with severe disease during admission, and tend to have high inflammatory markers like lactate dehydrogenase, C-reactive protein.15,16,31
Other risk factors for pulmonary fibrosis post COVID-19 include long intensive care unit (ICU) stay and IMV, smoking, obesity, chronic alcoholism, as well as shorter telomere due to its role in the development of fibrotic ILD [Table 2].32 It has been shown by many studies that the use of high flow oxygen could also contribute to pulmonary fibrosis.33
Patients with mild to moderate COVID-19 pneumonia may not be at risk for post-COVID-19 pulmonary fibrosis.32 The pathogenesis is a complex interplay between the virus and the immune response with downstream immune signaling activation.34 Pulmonary fibrosis results from abnormal repair of lung injury caused by various mechanisms including viral infections, inflammation, or idiopathic.21 In severe forms of lung injury due to COVID-19, the basement membrane becomes damaged and the repair process ends up with the formation of fibroblastic tissue and scarring, leading to architectural distortion and fibrosis.34 Although fibrotic phase is one of the pathological features of acute respiratory distress syndrome (ARDS),21 the exact reason why not all patients develop fibrosis remains unknown. Fibrosis post ARDS— unlike idiopathic pulmonary fibrosis (IPF)— does not have a prominent honeycombing pattern and does not progress over time. For that reason, the pathological findings in post-COVID-19 fibrosis seem to differ from that in IPF, the predominant difference being injury to the alveolar epithelial cells rather than to the endothelial cells.21,35
Thrombosis
COVID-19 may predispose patients to arterial and venous thrombosis (VTE) which is associated with high morbidity and mortality.36 The pathogenesis of thrombosis has been attributed to a complex interplay between excessive inflammation, platelet activation, endothelial dysfunction, and stasis.37,38 However, more studies are required to understand the exact mechanism. The pathophysiology is related to intravascular hyperinflammation leading to microangiopathic endothelial damage.35 The risk of pulmonary embolism (PE) appears high, reportedly affecting up to one-third of COVID-19 patients who underwent CT pulmonary angiogram (CTPA).39 Interestingly, an epidemiological study that compared the incidence of venous and arterial thrombotic complications at 30 days, between hospitalized COVID-19 and influenza patients, reported thrombotic complications in 23% of COVID-19 patients against only 3.6% in influenza patients.39 These complications were observed more in those who were in ICU during their acute phase. A study from China compared the incidence of VTE in hospitalized COVID-19 patients with hospitalized community-acquired pneumonia (CAP) patients and reported no difference. However, the generalizability of this study was limited by a selection bias toward younger COVID-19 patients with fewer comorbidities.40 Moreover, a case series of a population cohort from Scotland demonstrated significantly increased risk of myocardial infarction (MI) and ischemic stroke along with VTE.41 It was also noted that the VTE risk persisted longer than the arterial thrombotic complications did.
In one cohort of patients followed-up at six weeks after recovering from COVID-19 with pneumonia, 2% had previously undiagnosed PE that could have either been missed or developed post discharge [Figure 2].42 Cheng Fang et al,35 investigated all CTPA conducted among COVID-19 patients in a tertiary care center in China, found 40% to have evidence of PE, mostly at segmental and subsegmental levels. PE was more likely to occur in the second or third week of disease onset. D-dimer and Wells criteria were found not useful to predict who had higher risk of PE.35 The European Respiratory Society (ERS) guidelines strongly recommend that patients hospitalized with COVID-19 should be offered a form of anticoagulant therapy despite the low-quality evidence.43 Although the evidence for providing extended anticoagulation post-discharge is lacking, it can still reduce the VTE risk, albeit at the risk of increased bleeding. To minimize the latter, prophylactic anticoagulant treatment should be considered case-by-case in patients with high VTE risk and low bleeding risk.42 Additionally, patients who develop PE during the acute phase should be followed-up at three months post discharge with echocardiography and CTPA.44 If there is no evidence for residual thromboembolic disease and/or pulmonary hypertension, then anticoagulation can be discontinued.
Other respiratory complications
There are several other respiratory complications secondary to COVID-19. Bronchiectasis, which in general carries a poor prognosis, has been reported after severe COVID-19, and could be either due to the disease itself or secondary to superimposed bacterial infection.45 There is limited data about bronchiectasis in patients who survived COVID-19. However, with the knowledge that infection is the most common cause of bronchiectasis, we can expect many cases of post-COVID-19 bronchiectasis in the future. Studies on survivors of SARS showed that in the small number of patients who developed bronchiectasis, it evolved over long periods of follow-up.46,47 In a study from China on 81 patients post COVID-19, 11% had evidence of bronchiectasis.48
Cavitary lung disease is another complication of COVID-19. Selvaraj et al,49 reported a case of post-COVID-19 bilateral lung cavities where other etiologies were negative. A study from UAE showed that 7% of patients admitted with COVID-19 pneumonia developed cavitation, most of whom were ICU patients.50 The cavities were either single or multiple with sizes between 3–10 cm with thick smooth walls and fluid level.
Pneumothorax has been reported in about 1% of hospitalized COVID-19 patients irrespective of whether they were ventilated or not.51 The overall survival of those who developed pneumothorax was about 63%. Age > 70 carried a poor prognosis.52 Ufuk et al,52 reported a case of spontaneous pneumothorax in a COVID-19 patient three months after discharge.Another case of delayed recurrent pneumothorax was found four weeks after discharge.53 The mechanism for the development of delayed pneumothorax is not fully understood but could be related to damage of the alveolar walls due to the ongoing inflammatory process, or the formation of small alveolar blebs. Vigorous cough may also be a contributing factor.54 Therefore, physicians should consider the possibility of spontaneous pneumothorax in patients who recovered from COVID-19 pneumonia if their respiratory status deteriorates suddenly. It should be managed according to the established guidelines for secondary spontaneous pneumothorax.
Post-COVID-19 pulmonary fibrosis: Treatment and prevention
Currently, there is no proven treatment for post- COVID-19 pulmonary fibrosis. Many ongoing studies are investigating different treatment options. In a small single-center prospective observational study, 30 patients who had severe COVID-19 pneumonia and remained symptomatic at six weeks post discharge with impaired PFT and features of organizing pneumonia on CT chest, were offered prednisone 0.5 mg/kg for three weeks. There was a clear improvement in the dyspnea score with increase FVC by 9.6% and in TLCO by 31.5%. There was also significant improvement in the six-minute walk test (6MWT).14 The repeat chest CT scan also demonstrated remarkable improvement without any major complications from steroid use.14 The question remains whether this improvement was due to the steroid treatment or part of the normal recovery trajectory of the lung.
Saha et al,55 reported three cases of post H1N1 ARDS related fibrosis. After discharge the three patients were treated with a combination of prednisolone, azithromycin and pirfenidone for up to one year, and there was improvement in oxygenation, 6 MWT and HRCT changes.56 Further research is required to understand the exact mechanisms as well as the effectiveness of various early intervention measures. The current thinking is that early intervention during severe pneumonia may decrease the post-COVID-19 complications, though the best intervention is still not clear.25 The two approved antifibrotic medications (pirfenidone and nintedanib) to treat IPF has been proven to decrease lung function decline.56,57 For post- COVID-19 fibrosis, the current postulation is to start antifibrotic medications early. However, the use of pirfenidone at the acute phase may lead to hepatic toxicity, while nintedanib is associated with high risk of bleeding.21 Ongoing trials are investigating the roles of both in post-COVID-19 pulmonary fibrosis. Phase III NINTECOR (NCT04541680) and phase IV ENDCOV-I trials (NCT04619680) are studying the effects of nintedanib 150 mg twice daily on the changes in post-COVID-19 pulmonary fibrosis patient’s FVC. Pirfenidone is undergoing phase II (NCT04607928) and phase III
(NCT04282902) trials.58
Since there is currently no effective treatment for post-COVID-19 pulmonary fibrosis, the risk of fibrosis can be reduced by protective measures to minimize ventilator associated lung injury (VALI), such as lung protective ventilation and cessation of smoking.16 Furthermore, rehabilitation may be considered early before discharge as it may improve respiratory function despite the lack of
strong evidence.21
Lung transplant has become a treatment option for a wide variety of end-stage lung diseases. Bharat et al,59 reported the success of the first two US lung transplantations which were performed in patients with severe fibrosis related to COVID-19. It was reported that both patients had excellent results and were able to be free of any oxygen support after 4-8 weeks post transplantation.59 In addition, a recent case series of 12 patients who had bilateral lung transplantations in US, Italy, Austria, and India for post severe COVID-19 ARDS that did not improve despite prolonged ventilation and extracorporeal membrane oxygenation (ECMO), showed that transplantation had similar odds of survival as in non-COVID-19 patients.60 The authors recommended that patients can be listed for lung transplant if they spent more than four weeks in ICU and showed no signs of lung recovery despite full medical care, provided they fulfill the usual criteria for lung transplantation.60 The challenges in transplant surgeries include: the risk of a repeat COVID-19 infection in the transplanted lungs, ventilator related infections, technical difficulties during surgery especially in patients who required pleural procedures, and severe deconditioning due to prolonged ICU stay. Finally, it is still uncertain whether the native lung might eventually recover and outlive the transplanted lung.59
Recommendations
The current BTS guidelines recommend a follow-up CXR at 4–6 weeks post discharge for patients who were admitted with severe COVID-19 pneumonia— whether managed in ICU, high dependency unit or ward— and at 12 weeks for those who had non-severe COVID-19 pneumonia.30 It also recommends that if there is no improvement in CXR changes or the respiratory symptoms persist, then a full PFT, 6MWT, echocardiography, sputum for microbiology, and referral for rehabilitation are to be considered. If the CXR changes persist and there is physiological impairment, HRCT and CTPA should be considered.29 A chest physician should follow-up patients with post-COVID-19 complications.61 In this context, it should be noted that the role of bronchoscopy, with or without lung biopsy, for patients who have persistent or new radiological changes post-COVID-19 pneumonia, remains unclear.62
Conclusion
COVID-19 disease survivors, especially those who experienced severe acute phase, may develop complications which are associated with high morbidity and mortality. Currently, there is a lack of specific treatment for most of the post- COVID-19 lung complications. More long-term follow-up studies are warranted to determine the exact prevalence and prognosis of various post-COVID-19 complications, and to what extent the persistent ones such as fibrosis are permanent or reversible. Future may see evidence-based revelation of their mechanisms and increasingly effective treatment choices. As of now, clinical, physiological, and radiological monitoring and early referral to chest physicians are recommended for all COVID-19 survivors.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- World Health Organization. WHO director-general’s remarks at the media briefing on 2019-nCoV on 11 February 2020. 2020 [cited 2021 June 10] Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020.
- 2. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021 Jan;397(10270):220-232.
- 3. Townsend L, Dowds J, O’Brien K, Sheill G, Dyer AH, O’Kelly B, et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc 2021 Jun;18(6):997-1003.
- 4. Hall J, Myall K, Lam JL, Mason T, Mukherjee B, West A, et al. Identifying patients at risk of post-discharge complications related to COVID-19 infection. Thorax 2021 Apr;76(4):408-411.
- 5. Galal I, Hussein AA, Amin MT, Saad MM, Zayan HE, Abdelsayed MZ, et al. Determinants of persistent post-COVID-19 symptoms: value of a novel COVID-19 symptom score. Egypt J Bronchol 2021;15(1).
- 6. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med 2021 Apr;27(4):601-615.
- 7. Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open 2021 May;4(5):e2111417.
- 8. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020 Aug;324(6):603-605.
- 9. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2021 Apr;76(4):405-407.
- 10. Sigfrid L, Drake TM, Pauley E, Jesudason EC, Olliaro P, Lim WS, et al. Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol. Lancet Reg Health Eur 2021 Sep;8:100186.
- 11. Jiang Y, Guo D, Li C, Chen T, Li R. High-resolution CT features of the COVID-19 infection in Nanchong City: Initial and follow-up changes among different clinical types. Radiol Infect Dis 2020 Jun;7(2):71-77.
- 12. Fabbri L, Moss S, Khan F, Chi W, Xia J, Robinson K, et al. Post-viral parenchymal lung disease of COVID-19 and viral pneumonitis: a systematic review and meta-analysis. medRxiv 2021.
- 13. Zhang P, Li J, Liu H, Han N, Ju J, Kou Y, et al. Correction: Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res 2020 Sep;8(1):34.
- 14. Myall KJ, Mukherjee B, Castanheira AM, Lam JL, Benedetti G, Mak SM, et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc 2021 May;18(5):799-806.
- 15. Yu M, Liu Y, Xu D, Zhang R, Lan L, Xu H. Prediction of the development of pulmonary fibrosis using serial thin-section ct and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol 2020 Jun;21(6):746-755.
- 16. Guler SA, Ebner L, Aubry-Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J 2021 Apr;57(4):2003690.
- 17. Faverio P, Luppi F, Rebora P, Busnelli S, Stainer A, Catalano M, et al. Six-month pulmonary impairment after severe COVID-19: a prospective, multicenter follow-up study. Respiration 2021;100(11):1078-1087.
- 18. Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 2021 Apr;299(1):E177-E186.
- 19. So M, Kabata H, Fukunaga K, Takagi H, Kuno T. Radiological and functional lung sequelae of COVID-19: a systematic review and meta-analysis. BMC Pulm Med 2021 Mar;21(1):97.
- 20. Wells AU, Devaraj A, Desai SR. Interstitial lung disease after COVID-19 infection: a catalog of uncertainties. Radiology 2021 Apr;299(1):E216-E218.
- 21. Rai DK, Sharma P, Kumar R. Post covid 19 pulmonary fibrosis. Is it real threat? Indian J Tuberc 2021 Jul;68(3):330-333.
- 22. Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology 2021 Jul-Aug;27(4):328-337.
- 23. RECOVERY: Randomised evaluation of Covid-19 therapy. [cited 2022 January 26]. Available from: https://www.recoverytrial.net/.
- 24. Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J 2020 Jun;55(6):2001217.
- 25. Vasarmidi E, Tsitoura E, Spandidos DA, Tzanakis N, Antoniou KM. Pulmonary fibrosis in the aftermath of the COVID-19 era (Review). (Review). Exp Ther Med 2020 Sep;20(3):2557-2560.
- 26. Crameri GA, Bielecki M, Züst R, Buehrer TW, Stanga Z, Deuel JW. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Euro Surveill 2020 Sep;25(36):2001542.
- 27. Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med 2020 May;52(5):jrm00063.
- 28. Méndez R, Latorre A, González-Jiménez P, Feced L, Bouzas L, Yépez K, et al. Reduced diffusion capacity in COVID-19 survivors. Ann Am Thorac Soc 2021 Jul;18(7):1253-1255.
- 29. George PM, Barratt S, Desai SR. British thoracic society guidance on respiratory follow up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia. Br Thorac Soc 2020;(May).
- 30. Shojaee A, Siner JM, Zinchuk A, Aryan Y, Kaminski N, Cruz CS. Viral pneumonia is associated with increased risk and earlier development of post-inflammatory pulmonary fibrosis. medRxiv 2021.
- 31. McGroder CF, Zhang D, Choudhury MA, Salvatore MM, D’Souza BM, Hoffman EA, et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax 2021 Apr:thoraxjnl-2021-217031.
- 32. Rogliani P, Calzetta L, Coppola A, Puxeddu E, Sergiacomi G, D’Amato D, et al. Are there pulmonary sequelae in patients recovering from COVID-19? Respir Res 2020 Oct;21(1):286.
- 33. Otoupalova E, Smith S, Cheng G, Thannickal VJ. Oxidative stress in pulmonary fibrosis. Compr Physiol 2020 Mar;10(2):509-547.
- 34. Chaudhary S, Natt B, Bime C, Knox KS, Glassberg MK. Antifibrotics in COVID-19 lung disease: let us stay focused. Front Med (Lausanne) 2020 Sep;7:539.
- 35. Fang C, Garzillo G, Batohi B, Teo JT, Berovic M, Sidhu PS, et al. Pulmonary thromboembolic disease in patients with COVID-19 undergoing computed tomography pulmonary angiography (CTPA): incidence and relationship with pulmonary parenchymal abnormalities. BMJ 2020.
- 36. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA 2020 Aug;324(8):799-801.
- 37. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020 Jul;18(7):1738-1742.
- 38. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020 Sep;41(32):3038-3044.
- 39. Stals M, Grootenboers M, van Guldener C, Kaptein F, Braken S, Chen Q, et al. Dutch COVID, Thrombosis Coalition (DCTC). Risk of thrombotic complications in influenza versus COVID-19 hospitalized patients. Res Pract Thromb Haemost 2021 Feb;5(3) .
- 40. Mei F, Fan J, Yuan J, Liang Z, Wang K, Sun J, et al. Comparison of venous thromboembolism risks between COVID-19 pneumonia and community-acquired pneumonia patients. Arterioscler Thromb Vasc Biol 2020 Sep;40(9):2332-2337.
- 41. Ho F, Man K, Toshner M, Celis-Morales C, Wong I, Sattar N, et al. COVID-19 infection and subsequent thromboembolism: a self-controlled case series analysis of a population cohort. medRxiv 2021.
- 42. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 2020 Jun;75(23):2950-2973.
- 43. Chalmers JD, Crichton ML, Goeminne PC, Cao B, Humbert M, Shteinberg M, et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. Eur Respir J 2021 Apr;57(4):2100048.
- 44. George PM, Barratt SL, Condliffe R, Desai SR, Devaraj A, Forrest I, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020 Nov;75(11):1009-1016.
- 45. Apriningsih H, Prabowo NA, Ardyanto TD, Pradana RF. Bronchiectasis as a sequealae from COVID-19. In: 4th International Conference on Sustainable Innovation 2020–Health Science and Nursing (ICoSIHSN 2020) 2021 Jan 16; Atlantis Press. p. 105-108).
- 46. Müller NL, Ooi GC, Khong PL, Zhou LJ, Tsang KW, Nicolaou S. High-resolution CT findings of severe acute respiratory syndrome at presentation and after admission. AJR Am J Roentgenol 2004 Jan;182(1):39-44.
- 47. Wu X, Dong D, Ma D. Thin-section computed tomography manifestations during convalescence and long- term follow-up of patients with severe acute respiratory syndrome (SARS). Med Sci Monit 2016 Aug;22:2793-2799.
- 48. José RJ, Manuel A, Gibson-Bailey K, Lee L. Post COVID-19 bronchiectasis: a potential epidemic within a pandemic. Expert Rev Respir Med 2020 Dec;14(12):1183-1184.
- 49. Selvaraj V, Dapaah-Afriyie K. Lung cavitation due to COVID-19 pneumonia. BMJ Case Rep 2020 Jul;13(7):e237245.
- 50. Zoumot Z, Bonilla MF, Wahla AS, Shafiq I, Uzbeck M, El-Lababidi RM, et al. Pulmonary cavitation: an under-recognized late complication of severe COVID-19 lung disease. BMC Pulm Med 2021 Jan;21(1):24.
- 51. Martinelli AW, Ingle T, Newman J, Nadeem I, Jackson K, Lane ND, et al. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur Respir J 2020 Nov;56(5):2002697.
- 52. Ufuk F, Yavas HG, Kis A. An unusual cause of spontaneous pneumothorax: post-COVID-19 pulmonary fibrosis. Am J Emerg Med 2021 May;S0735-6757(21)00371-5.
- 53. Kasturi S, Muthirevula A, Chinthareddy RR, Lingaraju VC. Delayed recurrent spontaneous pneumothorax post-recovery from COVID-19 infection. Indian J Thorac Cardiovasc Surg 2021:1-3.
- 54. Abushahin A, Degliuomini J, Aronow WS, Newman T. A case of spontaneous pneumothorax 21 days after diagnosis of coronavirus disease 2019 (COVID-19) pneumonia. Am J Case Rep 2020 Aug;21:e925787.
- 55. Saha A, Vaidya PJ, Chavhan VB, Achlerkar A, Leuppi JD, Chhajed PN. Combined pirfenidone, azithromycin and prednisolone in post-H1N1 ARDS pulmonary fibrosis. Sarcoidosis Vasc Diffus Lung Dis. 2018;35(1). doi:10.36141/svdld.v35i1.6393
- King TE, Bradford WZ, Castro-Bernadini S, Fagan EA, Glaspole I, Glassberg MK, et al. The ascend study: a randomized, double-blind, placebo controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis (IPF). Am J Respir Crit Care Med 2014;189:A6602.
- 56. Costabel U, Richeldi L, du Bois R, Raghu G, Azuma A, Brown KK, et al. Efficacy and safety of nintedanib in patients with idiopathic pulmonary fibrosis: Results of two 52-week, phase III, randomized, placebo-controlled trials (INPULSISTM). Pneumologie 2015;69(S 01).
- 57. Yo EC, Kadharusman MM, Karman AP, Louisa M, Arozal W. Potential pharmacological options and new avenues using inhaled curcumin nanoformulations for treatment of post-COVID-19 fibrosis. SRP 2021;12(1):1119-1128.
- 58. Bharat A, Querrey M, Markov NS, Kim S, Kurihara C, Garza-Castillon R, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med 2020 Dec;12(574):eabe4282.
- 59. Bharat A, Machuca TN, Querrey M, Kurihara C, Garza-Castillon R Jr, Kim S, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med 2021 May;9(5):487-497.
- 60. Ojo AS, Balogun SA, Williams OT, Ojo OS. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies. Pulm Med 2020 Aug;2020:6175964.
- 61. Lutchmansingh DD, Knauert MP, Antin-Ozerkis DE, Chupp G, Cohn L, Dela Cruz CS, et al. A clinic blueprint for post-coronavirus disease 2019 recovery: learning from the past, looking to the future. Chest 2021 Mar;159(3):949-958.