|
Abstract
Objective: To study the clinicopathological findings of cylindrical cell papilloma (oncocytic schneiderian papilloma) diagnosed among patients at Bahrain Defense Force Hospital with review of literature.
Methods: All cases of cylindrical cell papilloma were retrieved from the pathology register from 2006-2010. The medical records of the cases were studied for age, sex, clinical presentation, and imaging reports along with the histopathological findings.
Results: Five cases were found and showed male to female ratio 4:1. Their ages were between 36-71 years with average age of 55 years. The main symptom found was unilateral nasal obstruction mainly involving the left side. All papillomas were removed by endoscopic sinus surgery. Four patients had regular followup with complete recovery, while only one case was lost for followup.
Conclusion: Cylindrical cell papilloma is a variant of sinonasal schneiderian papilloma. Although most are benign complete excision is the treatment of choice to prevent tumour recurrence as a proportion exhibit unpredictable biological behavior. This study is an important reminder for both clinicians and pathologists to recognize this tumor and to differentiate it from other tumours and rhinosporidiosis. Regular followup is an important strategy for complete recovery and tumour-free surveillance.
Keywords: Oncocytic schneiderian papilloma; Cylindrical cell papilloma; Sinonasal tumours; Inverted papilloma; Rhinosporidiosis.
Introduction
Schneiderian papilloma was first described by Ward in 1854 and it is believed to originate from the Schneiderian membrane which lines the sinonasal tract.1,2 In 1971, Hyams in his large clinicopathological series from Arm Force Institutes of Pathology was the first one who subclassified sinonasal papillomas into three subtypes: cylindrical, fungiform, and finally, inverted papillomas.3,4
These tumors are uncommon and represent only 0.4% to 4.7% of all sinonasal tumors.5 They often exhibit overlapping papillary and/or glandular features, on histological examination. As a result, they may be confused or even mistaken for metastatic tumors.6 Because of their rarity, Schneiderian papillomas are interesting lesions that have been the subject of clinicopathological and therapeutic controversy.6 This study is an important reminder for both clinicians and pathologists to recognize this tumor and to differentiate it from cancers such as adenocarcinoma and non-keratinizing squamous cell carcinoma as well as infectious diseases such as rhinosporidiosis. Cylindrical cell papilloma (CCP), also known as oncocytic schneiderian papilloma (OSP) and columnar cell papilloma, is the rarest of the three morphologic variants of schneiderian papillomas.4
In this paper, clinicopathological features of five cases of cylindrical cell papilloma diagnosed at Bahrain Defence Force (BDF) hospital are presented. The clinical presentation, diagnosis and treatment are discussed with literature review.
Methods
This study is a retrospective case series type conducted at Bahrain Defense Force Hospital – Royal Medical Services (BDF-RMS) in the Kingdom of Bahrain. It included patients who underwent endoscopic sinus surgery (ESS) for sinonasal polypectomy from January 2006 to December 2010. All cases of cylindrical cell papilloma diagnosed at that period were included in the study. These patients were assessed and managed by ENT surgeon (second author) at BDF-RMS. All patients' data including age, sex, clinical presentation and radiological reports were obtained through studying their medical records. The histopathological slides along with the pathology report were evaluated for further diagnostic confirmation by a qualified pathologist (first author).
This is a retrospective study and has a minimal ethical concern. It did not interfere with the patients’ management and only the research team had access to patients’ data base and information. Patients’ names were not needed in any part of the study or to access further information. However, prior to conduction of this study, BDF-RMS Ethical Committee approval was obtained as part of the process. A full clinical detail of the five cases is described in the following paragraphs:
Case 1
A 69-year-old male, presented to ENT clinic with a history of nasal obstruction on the left side for four months. He was a known smoker. Examination findings showed a polyp on the left side of the nose causing complete obstruction. The rest of the ENT examination was normal. CT Scanning of the paranasal sinuses showed a polypoidal mass about 2.8 cm centered at the left maxillary sinus protruding via the osteum into the nasal cavity and blended with the middle turbinate distorting the normal anatomy of the osteum. The remainder of the left maxillary sinus was opacified because of obstruction. There was no tumor extension into the orbit, the inferior rectus muscle was in normal position and the fat plane around it was preserved. There was a slight mucosal thickening at the right maxillary sinus, otherwise the rest of the sinuses were clear. Biopsy was taken and showed cylindrical cell papilloma. The patient refused surgery and was lost to follow up.
Case 2
A 60-year-old male, presented to ENT clinic with a history of left sided nasal blockage for seven years. On examination, a left-sided nasal polyp was noted. CT Scanning showed a polypoidal mass involving the left maxillary sinus and extending into the nasal cavity. Biopsy was taken for pathological examination which showed cylindrical cell papilloma. The patient underwent combined approach ESS and Caldwell-Luc for removal of papilloma. There was no sign of recurrence on postoperative followup at four years; however, he was found to have a nasal polyp on the right side which appeared to be inflammatory by histopathology examination.
Case 3
A 36-year-old male with a known case of renal transplant on immunosuppression therapy presented to ENT clinic with a history of severe nasal blockage, snoring, and sleep apnea. On examination, he was found to have severely hypertrophied inferior turbinates bilaterally obstructing the nasal airway. Fiberoptic examination of the postnasal space revealed a mass. CT scanning confirmed the physical examination findings. This patient underwent septoplasty, laser turbinectomy, and excision of the postnasal space mass. Histopathology examination showed cylindrical cell papilloma. His postoperative recovery was uneventful. He is currently being reviewed regularly in the clinic. There were no signs of recurrence at 6-year followup.
Case 4
A 71-year-old male, presented to the ENT clinic with a history of bilateral nasal obstruction for many years. On examination, he was found to have left sided nasal polyp. CT scanning of the sinuses showed left-sided nasal polyp suggestive of inverted papilloma. The patient underwent endoscopic sinus surgery on the left side with left-sided inferior turbinectomy. The pathological examination of the nasal polyp showed features of cylindrical cell papilloma. The patient's immediate postoperative period was uneventful. He was asymptomatic with no evidence of recurrence at 2-year followup.
Case 5
A 39-year-old female presented to the ENT clinic with right nasal blockage for several years. On examination, she was found to have right-sided nasal mass and clinical diagnosis of inverted papilloma was suspected. CT scan of the paranasal sinuses was performed with and without the use of IV contrast. There was a definite mass originating from the region of the superior and middle meati on the right side measuring 2.8 × 2.4 × 1.6 cm in dimensions. The mass appeared to compromise the right osteomeatal complex. The mass blocked the drainage of the right frontal sinus by direct extension with associated complete opacification of the sinus. No bone destruction or erosion was noted. Overall, the appearances were consistent with an inverted papilloma. The left ethmoid air cells, left frontal sinus, and both mastoid air cells were clear and well aerated. The nasal mass was sent for histopathological examination which showed features of cylindrical cell papilloma. The immediate postoperative period was uneventful. There were no signs of recurrence at 4-year followup.
Results
Five cases were found during the study period. The diagnosis of cylindrical cell papilloma was confirmed by studying the histopathological slides of the same cases by a qualified histopathologist (first author). Most of the patients were males with male-to-female ratio of 4:1. Their ages were between 36-71 years with average age of 55 years. The main symptom found was unilateral nasal obstruction mainly involving the left side. All papillomas were removed by endoscopic sinus surgery.
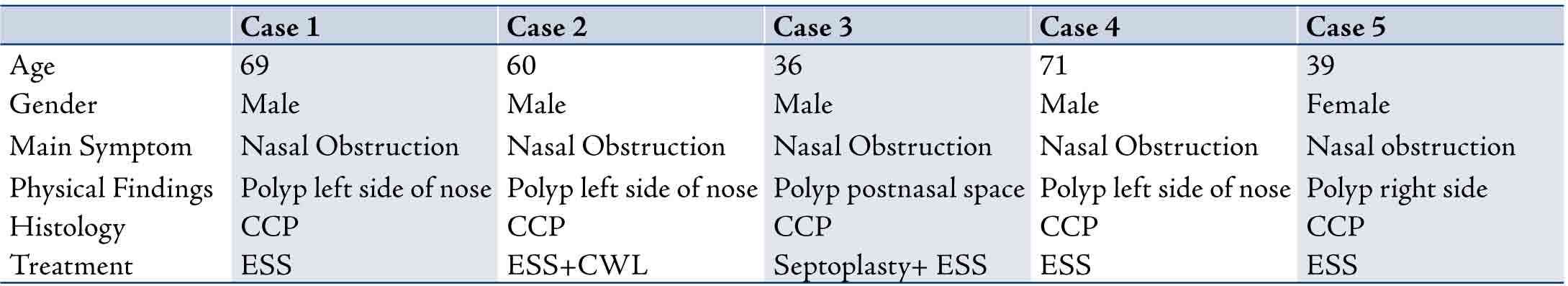
Table 1 shows the clinicopathological findings in five cases of cylindrical cell papilloma diagnosed in this study.
Table 1: Summary of the clinicopathological findings of the 5 cases described in this study. CCP: Cylindrical Cell Papilloma; ESS: Endoscopic Sinus Surgery; CWL: Caldwell Luc.
Discussion
Although there are no reliable data to estimate the incidence of schneiderian papillomas in the general population, they are relatively uncommon. They accounted for 25% of nasal tumors seen in the Institute of Laryngology and Otology in London.7 Vrabec and Suh et al. reported large series of 101 and 57 cases respectively of inverted papilloma over a 25- to 30-year study period.8,9
Cylindrical cell papilloma (CCP), also known as oncocytic schneiderian papilloma (OSP), is a rare benign sinonasal neoplasm. The frequency was found to be 6% of all schneiderian papillomas.5 The rarity of this tumor is further demonstrated in the presented case series where only five cases have been detected in the Bahrain Defense Force Hospital – Royal Medical Services (BDF-RMS) archives over the last 10 years. Due to its rare incidence, this disease is not well understood and is of interest to both clinicians and histopathologists. The probability of malignancies arising in CCP is not low, but they are rarely encountered in clinical practice.10
No known etiologic or risk factors were associated with the development of schneiderian papillomas in the past; however, in recent years Human Papilloma Virus (HPV) DNA has been detected in exophytic and inverted types using in situ hybridization and polymerase chain reaction (PCR).10 HPV 6 and 11 were the most common type detected.11 However, no association has been found between HPV and CCP.11,12
Sinonasal papillomas occur in a wide age range, but most cases are seen between 6 and 85 years of age.3,13-16 They are uncommon in children and the youngest patient reported in the literature was 6 years old.13 CCP occurs in both men and women. Males are affected twice as often as females.17 The majority of patients were above 50 years of age at the time of diagnosis. The five cases in this study were four males and one female (M:F ratio 4:1), their ages were between 36 to 71 years with an average age of 55 years. The main symptoms found in the previous studies at presentation include unilateral nasal obstruction and stuffiness, and less commonly epistaxis, facial pain and purulent discharge.3,14,18,19 Proptosis is generally seen with extensive bony erosion,18 but none of the patients in this study showed this feature. However, involvement of the middle ear and mastoid has been rarely described.20-23 In general, papillomas are unilateral3,13,19,24 and can be multifocal but rarely bilateral.25-28 However, the main presentation in our series was nasal obstruction with main involvement of the left side of the nose. In this case series, 4 out of 5 cases had unilateral nasal involvement and only one patient had OCP originating in the postnasal space. In one study of 72 patients with sinonasal schneiderian papilloma, the most common sites of involvement were the maxillary antrum (58.3%), lateral nasal wall (41.7%) and ethmoid sinus (37.5%).19 In the present case series, 2 patients had tumor involving the maxillary sinus and protruding into the nasal cavity, another 2 patients had tumor originating from the lateral nasal wall with no sinus involvement, and one patient had tumor originating in the postnasal space.
Oncocytic schneiderian papilloma (OSP) does not have a distinguished appearance on computer tomography (CT) and magnetic resonance imaging (MRI) and cannot be differentiated from other types of schneiderian papilloma based on imaging appearance.29 On CT, the tumors appear isoattenuated in comparison to the normal nasal mucosa. Bony erosion or remodeling due to pressure effect of the tumor on the adjacent bone may be seen but is not specific to OSP (CCP).30 CT scanning was done in all five cases which confirmed the presence of unilateral nasal masses; largest nasal papilloma measured 2.8 cm. MRI was not done in the case series; however, MRI has not been shown to facilitate differentiation between schneiderian papilloma and other tumors, and therefore its benefit would be to define tumor extent.31
The papillomas present as firm, bulky, red and vascular masses, usually on one side of the nose.3,32 It usually arises in the antrum and lateral nasal wall. It more often arises in the antrum without involvement of the lateral nasal wall than does the inverted papilloma. The appearance to the naked eye is of a finely granular surface.32 Microscopically, they are lined with multilayered epithelial proliferation of tall columnar cells with eosinophilic, granular cytoplasm. Scattered mucous cells with intraepithelial mucous cysts are also commonly seen.3,18 Architecturally, they also resemble inverted papilloma due to their endophytic growth pattern. The surface of these papillomas can also be lined by respiratory-type epithelium and frequently they also have transitional epithelial cell type component.33 CCP appears to have a similar relationship to malignancy as the inverted papilloma.34
One study stated that light microscopic, histochemical, immunocytochemical (cytochrome c oxidase), and ultrastructural studies can provide conclusive evidence.35 It has been found that the swollen, granular, eosinophilic epithelial cells in CCP are true oncocytes that arise primarily from the respiratory (Schneiderian) epithelium of the sinonasal passages rather than from minor salivary glands.35 Moreover, it has been suggested that fine needle aspiration may provide an immediate diagnosis.36 Hematoxylin and eosin (H&E) stain of high-quality tissue sections are adequate for morphology determination and pathology analysis. H&E appearance is sufficient to make a firm diagnosis although ancillary tests can help to confirm the results.
Beside other types of schneiderian papillomas, the differential diagnosis of CCP (OSP) includes a low-grade sinonasal papillary adenocarcinoma, non-keratinizing squamous cell carcinoma and rhinosporidiosis.18,37-39 Low grade sinonasal papillary adenocarcinoma is composed of infiltrative small acini and cribriform structures not seen in CCP.18 The surface epithelium of papilloma is usually thickened while in adenocarcinoma is usually normal.18 However, the degree of cytological atypia seen in carcinoma is not seen in papilloma. In the case of non-keratinizing SCC there is a degree of cytological atypia which is not seen in papilloma uncomplicated by concurrent carcinoma.18 Attention to other features such as inverted pattern, invasive glandular elements and pattern of tumour stroma is necessary to establish a definitive diagnosis.18 Pathologists must be aware of this appearance and so multiple representative tumour blocks and sections must be examined to identify the correct tumor type and diagnosis.32 Rhinosporidiosis must be taken as a differential diagnosis of nasal polypoid lesions.37 It is an infection caused by Rhinosporidium seeberi characterized by the presence of pseudotumoral lesions of the mucosa with polypoid, papillomatous, or verrucous aspect.37,38 It is friable, bloody to the touch, painless and the septum implantation is the most common form.37 The diagnosis is established on the morphological basis by the identification of 5- to 10-microm endospores and 50- to 1000-microm sporangia.38 Hematoxylin-eosin stained slides with the aid of Grocott special staining are used to make a diagnosis of rhinosporidiosis on microscopic examination.39
Surgical resection is the treatment of choice for all sinonasal papillomas; however there is no general agreement on the extent or type of surgery required.18 This approach was used in dealing with the cases presented here as all CCP (OSP) were removed by endoscopic sinus surgery (ESS). Most authors agree that recurrence of papilloma is a reflection of incomplete removal and recommend complete resection through a lateral rhinotomy incision with medial maxillectomy as adequate treatment.18 On the other hand, the endoscopic endonasal approach offers reduced morbidity in comparison to external approaches and its good control of the disease.40 Mendenhall et al. concluded that postoperative radiotherapy is added if inverted papilloma is associated with squamous cell carcinoma.41 They also concluded that definitive radiotherapy may be used to successfully treat patients with incompletely resectable inverted papilloma.
Therefore, a regular followup is important to monitor patients from unexpected recurrence or malignant behavior in some cases.
Conclusion
In this report, the clinicopathological features of five cases of cylindrical cell papilloma (CCP) diagnosed in our institute are presented and compared with previous studies that have been reported in the literature. This study is an important reminder for both clinicians and pathologists to recognize this tumor and to differentiate it from cancers such as adenocarcinoma and non-keratinizing squamous cell carcinoma as well as infections such as rhinosporidiosis. It is concluded that CCP is an uncommon sinonasal tumour with unpredictable behavior which may show both recurrence and malignant transformation if not completely excised. Pathologists and surgeons should be aware of this type of tumour as the treatment and regular followup strategy plays an important role in complete recovery and tumour-free surveillance.
Acknowledgement
The authors reported no conflict of interest and no funding has been received on this work.
References
1. Ward N. A mirror of the practice of medicine and surgery in the hospitals of London. London Hosp Lancet. 1854;2:480-482.
2. Ridolfi RL, Lieberman PH, Erlandson RA, Moore OS. Schneiderian papillomas: a clinicopathologic study of 30 cases. Am J Surg Pathol 1977;1(1):43-53.
3. Leon Barnes MD. Schneiderian Papilloma and Nonsalivary Glandural Neoplasms of the Head and Neck. Mod Pathol 2002;15(3):279-297.
4. Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. Ann Otol Rhinol Laryngol 1971;80:192-206.
5. Nwaorgu OG, Onakoya PA. Inverted papilloma of the nose and paranasal sinuses: a fifteen-year review. Afr J Med Med Sci 2002;31(3):191-194.
6. Low WK, Toh ST, Lim CM, Ramesh G. Schneiderian Papilloma of the nasopharynx. Ear Nose Throat J 2002;81(5):336-338.
7. Friedmann I, Osborn DA. Papillomas of the nose and sinuses. In pathology of Granulomas and Neoplasms of the nose and paranasal sinusesEdinburgh: Churchill Livingstone, 1982: 104-116.
8. Vrabec DP. The Inverted Schneiderian Papilloma: A 25-year study. Laryngoscope 1994;104:582-605.
9. Suh KW, Facer GW, Devine KD, et al. Inverting Papilloma of the nose and Paranasal Sinuses. Laryngoscope 1977;87:35-46.
10. Cheng TY, Ueng SH, Chen YL, Chang KP, Chen TM. Oncocytic Schneiderian Papilloma found in a recurrent chronic paranasal sinusitis. Chang Gung Med J 2006;29(3):336-341.
11. Cheung FM, Lau TW, Cheung LK, et al. Schneiderian papillomas and carcinomas: a retrospective study with special reference to p53 and p16 tumor suppressor gene expression and association with HPV. Ear Nose Throat J 2010;89(10).
12. Yoskovitch A, Frenkiel S, Franco E, et al. Analysis of human papillomavirus in schneiderian papillomas as compared to chronic sinusitis and normal nasal mucosa. J Otolaryngol 2001;30(3):167-172.
13. Eavey RD. Inverted papilloma of the nose and paranasal sinuses in childhood and adolescence. Laryngoscope 1985;95:17-22.
14. Díaz Molina JP, Llorente Pendas JL, Rodrigo Tapia JP, et al. Inverted sinonasal papillomas. Review of 61 cases. Acta Otorhinolaringol. 2009;60(6):402-408.
15. Dragonetti A, Gera R, Sciuto A, Scotti A, et al. Sinonasal inverted papilloma: 84 patients treated by endoscopy and proposal for a new classification. Rhinology 2011 Jun;49(2):207-213.
16. Olusina D, Nzegwu MA, Okoroafor IJ. Oncocytic Schneiderian papilloma occurring in a young Nigerian male: a case report. Ann Afr Med 2008;7(2):91-93.
17. Lawson W, Ho BT, Shaari CM, et al. Inverted Papilloma: A report of 122 cases. Laryngoscope 1995;105:282-288.
18. Douglas R. Gnepp: Nonsquamous Lesions of the Nasal Cavity and Paranasal Sinuses, and Nasopharynx. Diagnostic Surgical Pathology of the Head and Neck, 2001; 95-96.
19. Yoskovitch A, Braverman I, Nachtigal D, et al. Sinonasal schneiderian papilloma. J Otolaryngol 1998;27(3):122-126.
20. Seshul MJ, Eby TL, Crow DR, et al. Nasal inverted papilloma with involvement of the middle ear and mastoid. Arch Otolaryngol Head Neck Surg 1995;121:1045-1048.
21. Shen J, Baik F, Mafee MF, et al. Inverting papilloma of the temporal bone: case report and meta-analysis of risk factors. Otol Neurotol 2011 Sep;32(7):1124-1133.
22. Kainuma K, Kitoh R, Kenji S, et al. Inverted papilloma of the middle ear: a case report and review of the literature. Acta Otolaryngol 2011 Feb;131(2):216-220.
23. Zhou H, Chen Z, Li H, et al. Primary temporal inverted papilloma with premalignant change. J Laryngol Otol 2011 Feb;125(2):206-209.
24. Alba García JR, Díaz Fernández A, Rausell Fontestad N, et al. Inverted papilloma of paranasal sinuses. Our experience of 15 cases. Review of literature. An Otorrinolaringol Ibero Am 2003;30(2):137-149.
25. Jurlina M, Prstačić R, Zižić-Mitrečić M, et al. Synchronous multicentric bilateral sinonasal inverted papilloma and frontal sinus osteoma. J Craniofac Surg 2011;22(3):1114-1116.
26. Salomone R, Matsuyama C, Giannotti Filho O, et al. Bilateral inverted papilloma: case report and literature review. Braz J Otorhinolaryngol. 2008;74(2):293-296.
27. Rodriguez-Bruno K, Ali MJ, Wang SJ. Iatrogenic bilateral inverted papilloma: case report and literature review. J Otolaryngol 2007 Feb;36(1):72-75.
28. Yiotakis J, Hantzakos A, Kandiloros D, et al. A rare location of bilateral inverted papilloma of the nose and paranasal sinuses. Rhinology 2002;40(4):220-222.
29. Jacqueline A. Wieneke, Kelly K. Koeller. Head and Neck Radiology Pathology Classics. Head Neck Pathol 2007;1:99-101.
30. Lee DK, Chung SK, Dhong HJ, et al. Focal Hyperostosis on CT of Sinonasal Inverted Papilloma as a Predictor of Tumor Origin. AJNR Am J Neuroradiol 2007;28:618-621.
31. Yousem DM, Fellows DW, Kennedy DW, et al. Inverted papilloma: evaluation with MR imaging. Radiology 1992;185:501-505.
32. Gaze M.N., Wilson J.A. Tumors of the Nose and Sinuses. Stell and Maran Head and Neck Surgery 4th ED. 2000; 379-380.
33. Douglas R Gnepp: Nonsquamous Lesions of the Nasal Cavity and Paranasal Sinuses, and Nasopharynx. Diagnostic Surgical Pathology of the Head and Neck; 96.
34. Kaufman MR, Brandwein MS, Lawson W. Sinonasal papillomas: clinicopathologic review of 40 patients with inverted and oncocytic schneiderian papillomas. Laryngoscope 2002 Aug;112(8 Pt 1):1372-1377.
35. Barnes L, Bedetti C. Oncocytic Schneiderian papilloma: a reappraisal of cylindrical cell papilloma of the sinonasal tract. Hum Pathol 1984;15(4):344-351.
36. Rodic N, Maleki Z. Cytomorphologic findings of Schneiderian papilloma: A case report. Diagn Cytopathol 2011 May 4. doi: 10.1002/dc.21711. [Epub ahead of print].
37. Crosara PF, Becker CG, Freitas VA, et al. Nasal Rhinosporidiosis: Differential Diagnosis of Fungal Sinusitis and Inverted Papilloma. International Archives of Otolaryngology. 2009;13(1):93-95.
38. Hussein MR, Rashad UM. Rhinosporidiosis in Egypt: a case report and review of literature. Mycopathologia 2005 Feb;159(2):205-207.
39. Brevis P, Morales E, Bravo JC, et al. A new case of rhinosporidiosis in Chile. Rev Iberoam Micol 2010 Oct-Dec;27(4):183-185. REMOVED HYPERLINK FIELD.
40. Pagella F, Giourgos G, Matti E, et al. Endoscopic treatment of maxillary inverted papilloma. Rhinology 2011 Sep;49(3):369-374.
41. Mendenhall WM, Hinerman RW, Malyapa RS, et al. Inverted papilloma of the nasal cavity and paranasal sinuses. Am J Clin Oncol 2007;30(5):560-563.
|