Mitral valve regurgitation (MR) may be classified as primary or secondary/functional mitral regurgitation (FMR) depending on the presence or absence of a primary mitral valve pathology causing the regurgitation. Dilated cardiomyopathy (DCM) is usually associated with variable degrees of FMR, due to mitral annular dilatation, which results in malcoaptation of the mitral valve leaflets, or due to fibrosis of the sub-leaflet components of the mitral valve apparatus related to the etiology of left ventricular (LV) dysfunction. The severity of FMR in DCM is related to the severity of the underlying pathology.
Severe mitral regurgitation is associated with incremental mortality in patients with LV systolic dysfunction,1,2 and this is evident both in patients with ischemic and non-ischemic cardiomyopathies.2
In patients with DCM and severe FMR, although the costs are financially burdening, efforts to improve the patients’ quality of life and decrease morbidity and mortality are still not satisfactory.
Management of FMR includes optimal medical therapy, cardiac resynchronization therapy (CRT), and mitral valve surgery. There are limited options beyond medical therapy and CRT in patients unsuitable for surgery. These measures depend on mechanical relief of MR without surgery and have ignited much interest.
Two techniques to decrease the degree of MR percutaneously currently are available: (i) Mitral-clip, and (ii) transcoronary venous mitral annuloplasty using the Carillon® Mitral Contour System®.
We describe the first use of the Carillon Mitral Contour System followed by staggered CRT-defibrillator (D) implantation for the first time in Oman in a patient with severe LV dysfunction as well as severe FMR.
Case report
Our patient was a 64-year-old, ex-smoker. He was not diabetic nor hypertensive and was referred from another hospital in 2008 for evaluation of exertional dyspnea. Echocardiography at the time showed global hypokinesia with very poor LV systolic function and an ejection fraction (EF) of 15.0% associated with severe MR. Coronary angiography showed normal coronary arteries [Figure 1].
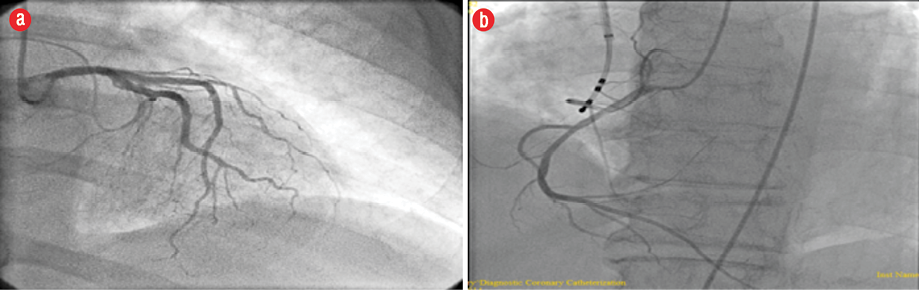
Figure 1: Coronary angiography revealed normal (a) left and (b) right coronary system.
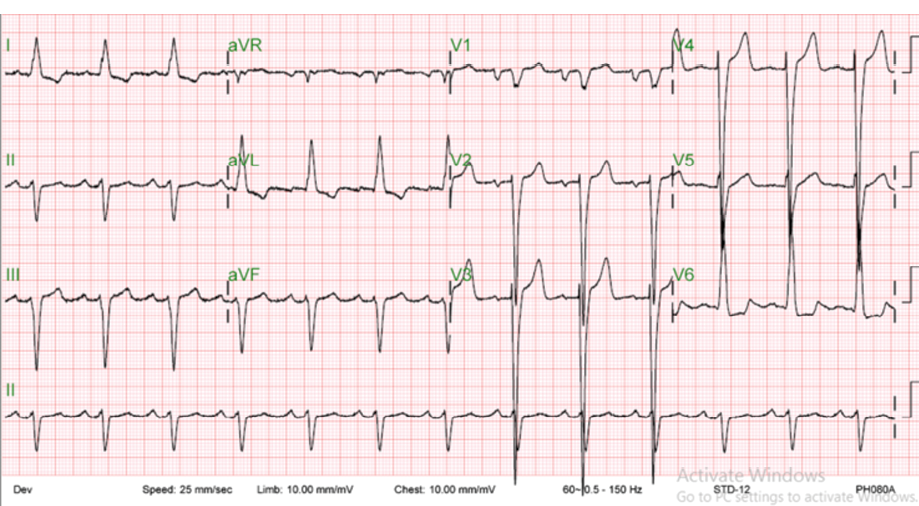
Figure 2: Echocardiogram of the patient before implantation of cardiac resynchronization therapy defibrillator showed sinus rhythm with left bundle branch block.
In April 2014, the patient’s symptoms were described as shortness of breath on minimal exertion, New York Heart Association (NYHA) functional class III-IV, orthopnea, and paroxysmal nocturnal dyspnea. Repeated echocardiogram (ECG) revealed the same findings of his previous ECG (i.e., global hypokinesia, EF of 15.0%, and severe MR). ECG showed normal sinus rhythm with left bundle branch block and wide QRS [Figure 2]. His 24-hour Holter ECG documented two episodes of non-sustained ventricular tachycardia.
Transesophageal echocardiography (TEE) showed severe MR with a dilated mitral annulus measuring 4.77 cm, vena contracta of 0.85 cm, and no structural abnormality of the mitral valve apparatus. After optimization of medical therapy, the patient was taken for a staged Mitral Contour System implantation followed by CRT-D device.
The Carillon device is a fixed-length double anchor implant with mirror-image hoop-shaped helical anchors that can be deployed in the coronary sinus (CS) to provide external splinting of the mitral annulus region to the maximal possible extent.
One month later, the patient was re-admitted, and a Boston Scientific Inogen X4 CRT-D was implanted under general anesthesia as requested by the manufacturing company to avoid any possible dislodgement of the previously deployed device.
The Carillon Mitral Contour System was implanted using right internal jugular vein access and a diagnostic electrophysiological catheter for CS access aided by CS venography, TEE, and fluoroscopy [Figure 3]. Traction was then applied to the delivery system, which facilitates plication of the perimitral annular tissue. The degree of traction applied and subsequent deployment of the second anchor within the CS ostium was guided by fluoroscopy, the patient was kept in the coronary care unit for observation, and discharged home three days after the procedure.
After six months with optimal medical therapy, the patient showed improvement in his general condition and his functional capacity increased.
Anti-failure measures (according to guidelines) were maintained until 2014 when he was referred back due to worsening of his symptoms.
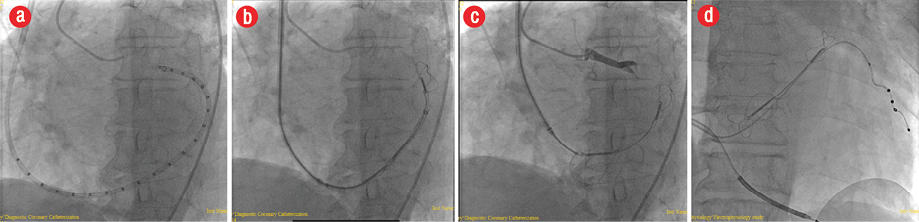
Figure 3: (a) Catheter insertion in the coronary sinus as seen by fluoroscopy. The Mitral Contour System as seen in the (b) proximal lobe and (c) proximal and distal lobes using fluoroscopy. (d) Fluoroscopy showed the Mitral Contour System proximal and distal lobes and the cardiac resynchronization therapy defibrillator leads after implantation.
Discussion
DCM is usually associated with variable degrees of FMR.2 MR has been shown to be an independent predictor of adverse outcomes in patients with post-infarction heart failure (HF) even to a mild degree.3
Advances in medical therapy combined with cardiac resynchronization have contributed to significant improvements in management; however, outcomes remain poor in individuals with advanced HF.4
Results from the Cleveland Clinic show an operative mortality of 2.3% in 44 patients with severe MR and severe LV dysfunction (EF < 35%) that underwent isolated mitral valve repair (MVR) or replacement.5 Surgical correction of MR is not an acceptable option in patients whose primary problem is a dilated failing heart.6 Additionally, cardiac transplantation is limited by donor availability so mechanical relief of MR without surgery becomes more interesting. There are two approaches to treat MR percutaneously. The first approach is to perform edge-to-edge (Alfieri) repair with a clip or suture, which opposes the centers of the two mitral leaflets producing a double-barrel opening for reducing the MR while avoiding mitral stenosis.7 The other approach is insertion of a device in the CS for attempting to mimic the effects of a surgically-placed annuloplasty ring.8
One of the designs for transcoronary venous mitral annuloplasty is the Carillon Mitral Contour System. The main limitations of percutaneous techniques for MVR using the transcoronary venous approach are the variability in coronary venous anatomy, the presence of venous valves within the coronary veins, and the variability of the crossing point of the great cardiac vein and circumflex artery.9
Our patient was not suitable for surgery and had a strong indication for CRT-D device implantation; the Mitral Contour System deployment approach followed by device implantation was ideal to utilize in this instance.
After six months, his functional class improved from NYHA class III-IV to class I. The patient’s exercise tolerance and mitral regurgitation parameters changed as follows:
- Decrease in effective regurgitant orifice from 0.70 to 0.40 mm2.
- Decrease in MR jet area ratio from 50.0% to 45.0%.
- Decrease in the mitral annulus size from 4.70 to 3.86 cm.
- Improved EF from 15.0% to 25.0%.
- Vena contracta unchanged (0.70 cm).
Conclusion
In selected high-risk cases with severe mitral regurgitation with severe LV systolic function the percutaneous mitral valve intervention using the Mitral Contour System can be a reasonable option for treatment with acceptable results.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J 2002 Sep;144(3):524-529.
- 2. Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003 Mar;91(5):538-543.
- 3. Lamas GA, Mitchell GF, Flaker GC, Smith SC Jr, Gersh BJ, Basta L, et al; Survival and Ventricular Enlargement Investigators. Clinical significance of mitral regurgitation after acute myocardial infarction. Circulation 1997 Aug;96(3):827-833.
- 4. Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005 Mar;352(9):875-883.
- 5. Enriquez-Sarano M, Loulmet DF, Burkhoff D. The conundrum of functional mitral regurgitation in chronic heart failure. J Am Coll Cardiol 2008 Jan;51(4):487-489.
- 6. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001 Nov;345(20):1435-1443.
- 7. Al-Amri HS, Al-Moghairi AM, El Oakley RM. Surgical treatment of functional mitral regurgitation in dilated cardiomyopathy. J Saudi Heart Assoc 2011 Jul;23(3):125-134.
- 8. Webb JG, Harnek J, Munt BI, Kimblad PO, Chandavimol M, Thompson CR, et al. Percutaneous transvenous mitral annuloplasty: initial human experience with device implantation in the coronary sinus. Circulation 2006 Feb;113(6):851-855.
- 9. Block PC. Percutaneous mitral valve repair: are they changing the guard? Circulation 2005 May;111(17):2154-2156.