Crohn’s disease (CD) is an inflammatory bowel disease (IBD).1–4 It is a chronic illness characterized by relapses and remissions.1–3,5,6 The etiology of CD is still unclear.1,3,7 However, our understanding of the etiology and pathogenesis of CD has significantly improved in the last decade.2 Genetic susceptibility, environmental factors, intestinal microbiota alteration, and disturbances in the innate and adaptive immune responses are the four main factors responsible for the disease.1–3,7–9 CD affects both adults and children.1 In around one-quarter of patients CD presents in childhood.6,7,10 The pediatric population represents a fertile source of new and useful data to help understand the triggers and pathogenesis of CD.4
The most common presentations of CD in pediatric IBD patients are abdominal pain and diarrhea.1,10–12 Abdominal pains may arise from different mechanisms which include intestinal distension secondary to the disease itself or partial blockage due to fibrotic stricture or adhesions as well as severe inflammation.12 Abdominal pain usually improves with decreasing disease activity, but the persistent pain in some cases suggests sensitization of the sensory pathway during inflammation.12,13 In addition, patients with CD are more likely to have weight loss and perianal disease.10,11 Weight loss in pediatric patients with CD is due to poor dietary intake, excessive gastrointestinal losses, and high energy expenditure due to chronic inflammatory activity.10,14 Compared to ulcerative colitis (UC), CD has a longer interval between symptom onset, signs, and diagnosis, and there is more systemic inflammation and therefore more effect on bone, appetite, and nutritional status.14,15 Because of malnutrition, pediatric patients with CD might be mistaken with patients with anorexia nervosa.10 Unlike adults with CD, the relapses of pediatric CD have a damaging effect on children’s linear growth and pubertal development.1,14 Short stature might be the only presenting sign of CD that can be recognized before the appearance of any gastrointestinal symptoms.10,14 A detailed nutritional assessment should be performed on a regular basis in all children and adolescents with CD.15 In addition to the gastrointestinal symptoms and signs, patients with CD are at risk of developing of extraintestinal manifestations like erythema nodosum and pyoderma gangrenosum, which might be linked to genetics, inflammatory cytokines, bacteria/bacterial products, or medications.1 The effect of CD on the musculoskeletal system ranges from mild arthralgia to severe spondyloarthropathies.1 Pediatric CD is a disorder that can lead to potential morbidities and lifelong challenges. It can leave both physical and psychosocial impact on children.1,4,10 The classical history, physical examinations, laboratory investigations, imaging, endoscopy, and histopathology are needed to diagnose pediatric CD.11
Western countries have the highest rate of CD, but the incidence and prevalence of the disease are increasing worldwide.10 In the Middle East, studies about pediatric CD are scarce. To date, there are no published studies about pediatric CD coming from Bahrain. The aim of this study was to review the prevalence, annual incidence, clinical presentations, means of diagnosis, therapeutic approaches, complications, and outcomes of pediatric patients with CD in Bahrain.
Methods
We conducted a retrospective review of all medical records of patients with CD diagnosed in the pediatric department, Salmaniya Medical Complex, Bahrain, between January 1984 and May 2017. All pediatric patients (≤ 18 years old) with CD were included in the study. We had no exclusion criteria. Despite being a single center study and in the absence of any population-based data, the annual incidence and cumulative prevalence of the disease were calculated. That was based on the fact that since established in 1979 to date, Salmaniya Medical Complex remained the only secondary and tertiary care hospital that covers the whole country and accepts to receive, diagnose, and manage pediatric patients with IBD. Moreover, it is the only health care facility that provides pediatric gastroenterology consultations in Bahrain. Data about gender, nationality, initial clinical presentation, age at presentation, age at diagnosis, duration of the illness, consanguinity, family history of IBD, contact with smokers, complications, number of exacerbations, number of hospital admissions, associated medical diseases, therapy, and outcome were collected.
Full blood count, erythrocytes sedimentation rate (ESR), C-reactive protein (CRP), liver function tests, antineutrophil cytoplasmic antibodies (ANCA), anti-saccharomyces cerevisiae antibodies (ASCA), helicobacter pylori serology and culture, purified protein derivatives (PPD) test, stool microscopy, and culture data were also collected. All results of radiological imaging studies including chest X-ray, abdominal ultrasound, small bowel series, abdominal computed tomography (CT), and magnetic resonance imaging (MRI) were reviewed. The Olympus (PCF-230 and XQ-230) or Pentax endoscopes (EG-2901 and EC-380IF) were used. Hematoxylin and eosin stain was used to read the gastrointestinal biopsies. After excluding ulcerative colitis, infective, and allergic causes, the diagnosis of CD was confirmed using clinical, biochemical, radiological, endoscopic, and histopathologic findings. The criteria published by IBD working group of the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) was adopted to confirm the diagnosis of CD.16,17 Data about medical therapy used to control CD, whether at the time of diagnosis or during the follow-up course, number of exacerbations, number of hospital admissions, complications, and outcomes were gathered.
This study was conducted in accordance with the principles of Helsinki Declaration and was ethically approved by the secondary care medical research subcommittee, Salmaniya Medical Complex, Ministry of Health, Bahrain.
The patients’ data were initially entered into Excel sheet then analyzed using SPSS Statistics (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp). The frequencies and percentages were calculated for demographic data. Continuous variables were explored for normal distribution using Kolmogorov-Smirnov and Shapiro-Wilk tests. Grouped data were presented as mean and standard deviation (SD) for normally distributed variables or median and range for non-normally distributed variables. Kruskal- Wallis test was used to compare the incidence between three time periods set every 10 years. A p- value < 0.050 was considered statistically significant.
Results
Of 108 pediatric patients diagnosed with IBD during the study period, 51 (47.2%) patients had CD. The prevalence of pediatric CD in Bahrain was 9.32 patients per 100 000 [Figure 1]. According to 2014 statistics, the population of Bahrain was 1 314 564, with 343 176 people aged less than 18 years. The estimated median annual incidence of CD was 1 in 100 000 per year (range = 0–5 patients/year). The median incidence of patients with CD was calculated based on the number of children during the previous period. These numbers were extracted from the Ministry of Health statistics. The median incidence was 0.006 per 100 000 per year (range = 0.0–0.56) between 1987 and 1997. The median incidence of patients with CD between 1998 and 2007 was 1.165 per 100 000 per year (range = 0.5–2.0) and the median incidence between 2008 and 2017 was 1.18 per 100 000 per year (range = 0.4–2.0). There was a significant statistical difference between the three time periods (p = 0.0001).
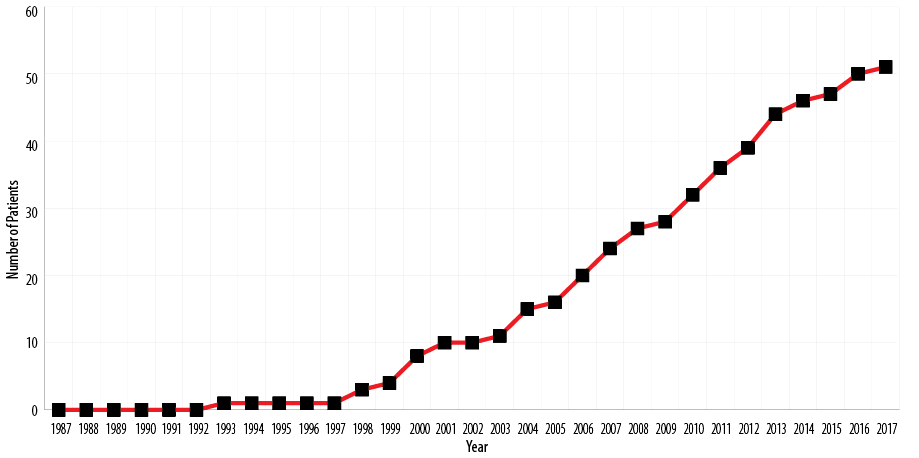
Figure 1: Cumulative prevalence of pediatric Crohn’s disease in Bahrain between 1987 and 2017.
Demographic data and clinical characteristics of pediatric patients with CD are presented in Table 1. Thirty-four patients (66.7%) were males, and 17 (33.3%) were females. Forty-six (90.2%) were Bahraini, and five (9.8%) patients were non-Bahraini (two children were from India, one from Jordan, one from Pakistan, and one from Syria). All patients were born normally with no history of lower segment cesarean section deliveries. The median age at the time of the study was 18.5 years (range = 6.4–35.0). Five (9.8%) patients had early onset disease. Family history of IBD was found in 10 (41.7%) out of 24 patients; seven had first-degree relatives while two and one had second and third-degree relatives, respectively. No associated chronic diseases were noted.
The most common initial clinical presentations were recurrent abdominal pain and weight loss [Table 2]. Other presentations were found in four patients. Erythema nodosum was found in two patients. Eye involvement, arthritis, and clubbing were each found in one patient. No patients had pyoderma gangrenosum.
Laboratory features at the initial presentation of the disease are shown in Table 3. Low hemoglobin level was found in 35 (79.5%) patients with a mean of 11.0±2.5 g/dL (normal range = 13.0–16.5 g/dL). Hypoalbuminemia was found in 26 (57.8%) out of 45 tested patients with a mean of 32.8±8.2 g/L (normal range = 35.0–50.0 g/L); and low vitamin D level in five out of 11 tested patients with a mean of 45.0±19.0 nmol/L (normal range > 50 nmol/L). ANCA test was performed in 11 patients, only one tested positive (9.1%). Four patients tested for ASCA and all were negative. Serum bilirubin and serum folate levels were normal in all tested patients. A PPD and chest X-ray were done to exclude tuberculosis as an alternative diagnosis that contraindicates the use of immune suppression in patients suspected to have IBD (specifically CD), both were done in all patients and all were negative.
Forty-two (82.4%) patients had at least one radiological imaging whether abdominal ultrasound, barium study, abdominal CT, or MRI scan to evaluate the small bowel; 35 (83.3%) had positive findings suggestive of CD [Table 4].
Table 1: Demographic data of 51 pediatric patients with Crohn’s disease.
|
Gender |
|
|
Male |
34 (66.7) |
|
Female |
17 (33.3) |
|
Nationality |
|
|
Bahraini |
46 (90.2) |
|
Non-Bahraini |
5 (9.8) |
|
Total population* (2014) n = 1 240 000 |
|
|
Governorate* Northern, n = 326 000 (26.3%) |
16 (31.4) |
|
Capital, n = 532 000 (42.9%) |
15 (29.4) |
|
Muharraq, n = 216 000 (17.4%) |
13 (25.5) |
|
Southern, n = 166 000 (13.4%) |
7 (13.7) |
|
Birth weight, mean ± SD, kg |
3.1 ± 0.4 |
|
Admission weight, mean ± SD, kg |
28.2 ± 9.7 |
|
Admission height, mean ± SD, cm |
136.5 ± 7.7 |
|
Age at presentation, median (range), years |
11.6 (0.4–18.0) |
|
Age, years |
|
|
0–4.9 |
5 (9.8) |
|
5–9.9 |
11 (21.6) |
|
10–14.9 |
23 (45.1) |
|
15–18 |
12 (23.5) |
|
Age at diagnosis, median (range), years |
11 (0.4–17.0) |
|
Disease duration, median (range), years |
0.17 (0.04–7.0) |
|
Family history of IBD** |
10 (41.7) |
*Adapted from: https://en.wikipedia.org/wiki/Governorates_of_Bahrain [cited 11 Jan 2017].
**Out of 24 patients.
SD: standard deviation; IBD: inflammatory bowel disease.
Table 2: Clinical presentations of 51 pediatric patients with Crohn’s disease.
|
Recurrent abdominal pain |
25 (49.0) |
|
Weight loss |
23 (45.1) |
|
Bloody diarrhea |
16 (31.4) |
|
Anorexia |
15 (29.4) |
|
Vomiting |
10 (19.6) |
|
Perianal disease |
8 (15.7) |
|
Arthralgia |
7 (13.7) |
|
Constipation |
6 (11.8) |
|
Pallor |
5 (9.8) |
|
Fever |
5 (9.8) |
|
Others |
4 (8.0) |
Table 3: Results of laboratory investigations of 51 pediatric patients with Crohn’s disease at presentation.
|
High WBC count |
8 (18.6) |
43 (84.3) |
|
Low Hb |
35 (79.5) |
44 (86.3) |
|
High platelets |
15 (34.9) |
43 (84.3) |
|
High ESR |
19 (51.4) |
37 (72.5) |
|
High CRP |
27 (71.1) |
38 (74.5) |
|
Low protein |
4 (9.1) |
44 (86.3) |
|
Low albumin |
26 (57.8) |
45 (88.2) |
|
High globulin |
40 (91.0) |
44 (86.3) |
|
High ALP |
1 (2.3) |
44 (86.3) |
|
High ALT |
4 (9.3) |
43 (84.3) |
|
High GGT |
2 (4.7) |
43 (84.3) |
|
Low serum iron |
12 (50.0) |
24 (47.1) |
|
Low serum ferritin |
11 (45.8) |
24 (47.1) |
|
Low vitamin B12 |
1 (5.0) |
20 (39.2) |
|
Low vitamin D |
5 (45.5) |
11 (21.6) |
WBC: white blood cells; Hb: hemoglobin; ESR: erythrocytes sedimentation rate; CRP: C-reactive protein; ALP: alkaline phosphatase; ALT: alanine aminotransferase; GTT: gamma-glutamyltransferase.
Of 51 patients with CD, 47 (92.2%) had endoscopic data available. Terminal ileum was the main area of involvement followed by the colon. Of 40 (78.4%) patients who had an upper gastrointestinal endoscopy, 19 (47.5%) had reports available, and 13 (68.4%) had positive findings. Esophageal involvement was noted in five patients (mild distal erythema in four patients and small nodules, aphthous ulcers, and small sliding hiatal hernia in one patient). Gastric involvement was found in 14 patients (diffuse erythema in four, multiple ulcers in four, macro-nodularity in two, congested mucosa in two, fundal varices in one, and dull mucosa in one patient). The patient with fundal varices had infantile onset CD as it was diagnosed before the age of six years (at six months specifically), which was managed with mesalazine. At the age of 16, he had an episode of acute abdomen, and CT angiography revealed thrombosis of both superior mesenteric artery and vein. The patient was kept on regular warfarin but developed fundal and rectal varices. Duodenal involvement was seen in six patients (severe erythema in three patients, multiple aphthous ulcers and nodularity shiny mucosa each in two patients, and dull mucosa and friable easy to bleed mucosa each in one patient).
All 51 patients with CD underwent colonoscopy, but reports were available for 44 (86.3%); 36 (81.8%) had positive findings. Perianal disease was noted in eight patients (perianal fistula in five and anal fissure in three; perianal granuloma and anal fissure in one of the eight patients was also found), multiple kissing ulcers covered with necrotic tissue with skipped lesion in nine, mucosal erythema in three, congested mucosa in two, and rectal varices and polyp in one patient each. The rectal varices were found in the same patient who had mesenteric artery and vein thrombosis. Terminal ileum reports were available for 14 patients; 10 were positive (ulcers in seven patients and cobble-stoning in three; one of the 10 patients had erythema, narrowing, edema, pseudopolyp and flat mucosa). No patient underwent capsule endoscopy.
Table 4: Radiological, endoscopic and histopathological investigations of 51 pediatric patients with Crohn’s disease.
|
Radiological |
|
|
|
Abdominal ultrasound |
17 (45.9) |
37 (72.5) |
|
Barium study |
24 (72.3) |
33 (64.7) |
|
Abdominal CT scan |
13 (92.9) |
14 (27.5) |
|
Abdominal and pelvic MRI scan |
1 (50.0) |
2 (3.9) |
|
Endoscopic |
|
|
|
OGD |
13 (32.5) |
40 (78.4) |
|
Colonoscopy |
36 (81.8) |
44 (86.3) |
|
Area of involvement |
|
|
|
Terminal ileum |
32 (68.1) |
47 (92.2) |
|
Colon |
30 (63.8) |
47 (92.2) |
|
Stomach |
24 (51.1) |
47 (92.2) |
|
Small bowel |
23 (48.9) |
47 (92.2) |
|
Esophagus |
19 (40.4) |
47 (92.2) |
|
Perianal disease |
8 (17.0) |
47 (92.2) |
|
Histopathological |
|
|
|
Upper gastrointestinal biopsies |
31 (91.2) |
34 (66.6) |
|
Colon and terminal ileum biopsies |
40 (95.2) |
42 (82.4) |
|
Chronic mucosal inflammation |
27 (67.5) |
40 (48.4) |
|
Non-caseating granuloma |
15 (37.5) |
40 (48.4) |
|
Cryptitis |
15 (37.5) |
40 (48.4) |
|
Focal non-specific ulcerations |
14 (35.0) |
40 (48.4) |
|
Crypt abscesses |
11 (27.5) |
40 (48.4) |
|
Prominent lymphoid follicles |
9 (22.5) |
40 (48.4) |
|
Crypt distortion |
7 (17.5) |
40 (48.4) |
CT: computed tomography; OGD: esophago-gastro-duodenoscopy; MRI: magnetic resonance imaging.
Upper gastrointestinal histopathology reports were available for 34 (66.6%) patients; 31 (91.2%) had positive results. Some patients had more than one histological finding in the same tissue biopsy. Nineteen (40.4%) out of 47 esophageal biopsies were positive (non-specific chronic active esophagitis in 12, mild papillary hyperplasia in four, basal cell hyperplasia in three, mild congestion in three, focal eosinophils infiltrate in two, and candida esophagitis, lymphocytic infiltrate and focal polymorphs infiltration each in one patient). Twenty-five (80.6%) out of 31 gastric biopsies were positive (non-specific chronic active gastritis in 22, noncaseating granuloma in two, helicobacter association in two; and mild congestion, foveolar prominence and cystic dilatation each in one). Twenty (71.4%) out of 28 duodenal biopsies were positive (chronic active duodenitis in 17 patients while ulceration, superficial mucosal necrosis, heavy infiltrates in lamina propria, mild excess in plasma cells, cryptitis, focal crypt abscess, and crypt hyperplasia each in one patient).
Colonic histopathology reports were available for 42 (82.4%) patients, 40 (95.2%) were positive. Chronic mucosal inflammation, noncaseating granuloma, cryptitis, and focal non-specific ulcerations were the main findings [Table 4]. Other findings were also seen in 11 (27.5%) patients such as focal mucodepletion in five, granulation tissue in four, mild increase in lymphoplasmacytic infiltrate in four; glandular distortion, focal hemorrhage, Paneth cell metaplasia, increase in mononuclear cells, and eosinophilic infiltrate each in two patients; and deep mucosal gap, mucosal congestion, inflammatory polyps, and neuromatoid proliferation each in one patient.
The most frequently used medications were prednisolone, azathioprine, and mesalazine [Table 5]. Biological therapy was used in five patients (22.7%). The indications to use biologics were either the presence of fistulizing CD (three patients) or poor response to conventional medical therapy (two patients). In four patients, the response was healing of the fistulae and induction of remission. One patient did not respond initially to adalimumab but responded well to infliximab. No patient received cyclosporine, methotrexate, or antituberculosis medications. No data were available about exclusive enteral nutrition or vitamin D supplementation.
Table 5: Medications used in the management of 22 pediatric patients with Crohn’s disease at the time of diagnosis and during follow-up.
|
Prednisolone |
16 (72.7) |
|
Azathioprine |
16 (72.7) |
|
Mesalazine |
12 (54.5) |
|
Pentasa |
7 (31.8) |
|
Asacol |
6 (27.3) |
|
Folic acid |
9 (40.9) |
|
Iron supplementation |
7 (31.8) |
|
Omeprazole |
6 (27.3) |
|
Calcium supplementation |
6 (27.3) |
|
Multivitamins |
6 (27.3) |
|
Biological therapy |
5 (22.7) |
|
Adalimumab |
4 (18.2) |
Table 6: The reported incidence and prevalence of pediatric Crohn’s disease in Middle Eastern countries.
|
Bahrain* |
Isa et al. 2018 |
1987–2017 |
< 18 |
1.0 |
9.32 |
|
Kuwait |
Al-Qabandi et al.19 2015 |
1998–2008 |
≤ 15 |
1.53 |
NR† |
|
Saudi Arabia, Riyadh |
El Mouzan et al.20 2006 |
1993–2002 |
< 18 |
0.5 |
NR |
|
Saudi Arabia |
El Mouzan et al.21 2014 |
2003–2012 |
0–14 |
0.27 |
NR |
|
Lebanon |
Abdul-Baki et al.22 2007 |
2000–2004 |
Pediatric and adults |
1.4 |
NR |
|
Turkey |
Tozun et al.23 2009 |
2001–2003 |
Pediatric and adults |
2.2 |
NR |
|
Libya |
Ahmaida et al.24 2009 |
1997–2006 |
< 15 |
NR |
2.0 |
*The present study.
†no record. To the best of our knowledge, no reports on epidemiology of pediatric Crohn’s disease were published from the following countries: Qatar, United Arab Emirates, Oman, Iraq, Iran, Yemen, Syria, Jordan, Egypt, Sudan, Algeria, or Morocco.
During the study period, eight patients had at least one CD relapse. The median number of relapse episodes per patient was two (range = 0–7 episodes). The median number of admissions per patient was four (range = 0–10 admissions). Out of 79 admissions, 17 (21.5%) were due to CD exacerbation. Three patients developed steroid dependence (5.8%). Three patients had anemia, which required a blood transfusion. Esophageal stricture, failure to thrive, short stature, pneumonia, septicemia, and mesenteric artery and vein thrombosis were noted in one patient each. Surgical interventions were required in six patients. Two patients had an inguinal hernia repair, and two had a tonsillectomy. Endoscopic esophageal stricture dilatation, appendectomy, perianal abscess drainage, and fistula repair were performed in one patient each. Gastrostomy was performed in one patient who had esophageal stricture secondary due to CD with severe dysphagia and failure to thrive. Total colectomy was required in one patient due to severe disease, short stature, and very poor response to medical treatment including biologic therapy. One patient had a post-infliximab anaphylactic reaction. The mean follow-up period was 9.2±5.6 years. No mortality was reported.
Discussion
The prevalence and incidence of pediatric CD are on the rise worldwide.10 This change in epidemiology could be attributed to environmental factors, urbanization across the globe, and the adoptation of westernized living styles.18 The prevalence of pediatric CD in our study was 9.32 per 100 000, which is lower than that reported in some western countries.14,18 Data on the epidemiology of pediatric CD from the Middle East are limited. The available data are summarized in Table 6.19–25 A study from Saudi Arabia of 455 patients with CD and found a striking increase in the prevalence of CD.11
In this study, the estimated median annual incidence of CD was 1 per 100 000 per year with a significant rise when comparing the three decades across the study period. The increasing incidence of CD in Gulf Cooperation Council countries may be related to the growing awareness of the disease.19,26 CD incidence peaks in the second and third decades of life (late adolescence and early adulthood).4 In our study, where only pediatric patients under the age of 18 years were involved, the median age at presentation was 11.6 years old.
Similar to our study, all of the original studies reviewed showed male predominance in patients with CD.11,19,26–28 Previous male to female ratios noted were 2:1 and 4.5:1.11,29 A family history of IBD in our study was noted in 41.7%. This is higher than the number reported from Kuwait (20%) and Saudi Arabia (13.6%).19,30
The clinical presentations of patients with CD in this study were similar to other studies where abdominal pain (49.0%), weight loss (45.1%) and bloody diarrhea (31.4%) were the most common presentations.6,11,12,15,19,26,30–32 However, the study from Kuwait reported a higher rate of abdominal pain (90%) and diarrhea (76%).19 The rate of weight loss we observed was lower than two previous studies (75.2% and 78%).30,33
In our study, eight (17.0%) patients had perianal diseases; five had perianal fistulae. Likewise, perianal diseases were reported in 17% of patients from Kuwait and 13% of patients from Saudi Arabia with a greater number (44%) reported in patients with CD from Libya.19,24,30
In this study, 13.7% of patients with CD had arthralgia. This is similar to a study from Saudi Arabia where the figure was 13.5%.28 The standard diagnostic laboratory tests used to diagnose CD in children are similar to those in adults.10 Although some pediatric patients with CD, especially those with mild disease, will have no laboratory abnormalities detected, the presence of iron deficiency anemia, elevated inflammatory markers (e.g., ESR, CRP and fecal calprotectin, hypoalbuminemia), and negative stool cultures are suggestive of CD.1,10 In our study, anemia, elevated inflammatory markers, and hypoalbuminemia were all on the higher side. Anemia was noted in 79.5% of our patients which was higher than that reported previously (25% and 57.9%).11,30 Studying the causes of the anemia was not the aim of the study. Iron deficiency anemia was documented in 45.8% of the 24 tested patients based on serum ferritin levels (50.0% of them had low serum iron). This make it inappropriate to attribute anemia to CD alone since other etiologies of anemia were not excluded. High ESR and CRP were noted in 51.4% and 71.1% of our patients, respectively. This was again higher than that reported in Saudi patients (25% and 18%).11 Hypoalbuminemia was seen in 57.8% of patients in this study compared to that a different study from Saudi Arabia, which was 34.5%.30
Although serological tests are available to screen for IBD, they fail to diagnose pediatric CD in at least 30% or give false positive results.1 ANCA was positive in only one (9.1%) of the 11 patients we tested while ASCA test was negative in all four tested patients. Similarly, positive ANCA was reported in 10.3% of patients (of a total 97) while ASCA was positive in 51% (of a total 92 patients).30
Radiological evaluation of children suspected to have CD should be individualized.1 Abdominal ultrasound can be used to detect CD in children with high success.31,34 Bowel wall thickening, peri-intestinal inflammation, and extraintestinal complications like fistula, abscesses, and ileus can be detected by transabdominal ultrasound.34 Although upper gastrointestinal study with small bowel follow-through is the main diagnostic radiological modality in patients with CD, the information obtained from its images to detect the presence or to see the extent of CD are limited.1 CT and MRI can provide better visualization of the inflamed mucosa and peri-intestinal involvement like abscesses or fistula.1,8,34 Our study showed higher positive findings of CD using abdominal CT scan (92.9%) compared to barium study (72.3%) or abdominal ultrasound (45.9%). However, the major disadvantages of CT scan are high exposure to radiation and the need for oral preparations.35
Ideally, all pediatric patients suspected to have CD should have an endoscopic procedure (upper and lower gastrointestinal endoscopy and biopsies) to confirm the diagnosis.7,30 All our patients had endoscopic procedures done to confirm the diagnosis, but none had capsule endoscopy. Although capsule endoscopy is not widely used in children at present, it may become increasingly valuable in the diagnosis of small bowel diseases in the future.8,31 In our study, the terminal ileum (68.1%) and the colon (63.8%) were the main areas involved. This is similar to that reported previously where the terminal ileum was involved in 72% and the colon in 68%.19 In this study, esophageal involvement was noted in 40.4% of patients, which is far higher than that reported from Kuwait where only 8% had an upper gastrointestinal involvement.19 This can be explained by higher number of upper gastrointestinal endoscopies performed to our patients (78.4%) compared to that in Kuwait (48%).19
Treatment of CD consists of medical and surgical approaches.2 Although medical advances have created drugs to control CD and improve quality of life, patients are at risk of infections due to the use of these immunosuppressive drugs or due to the disease.5 Medical treatment of children with CD should consider both induction and maintenance phases.1,2 In active pediatric CD, exclusive enteral nutrition (EEN) still has an important role as first-line therapy.14,36 In our study, no patient had received EEN. However, EEN is associated with early treatment failure probably due to its implications on normal daily life (e.g., nasogastric tube insertion and difficulties to sustain adherence).14 Most clinicians rely on corticosteroids for induction of rapid remission.1,2,12,14,36,37 Most of our patients (72.7%) received steroids for induction of remission. Similarly, a study in 188 patients with CD used steroids in 72.9% of patients.37 Previous studies have used steroids in almost all patients (97.4%)30 and in only 37% of patients.27 However, one-third of IBD patients will fail to respond to corticosteroids.2
Immunomodulators (e.g., azathioprine), aminosalicylates (e.g., mesalazine), and biologic agents (e.g., infliximab and adalimumab) are the main maintenance drugs used.1,2,12,14,36,37 Azathioprine was used in 72.7% of our patients. Previous studies have reported its use in 91.7% and 42.6% of patients.30,37 We report mesalazine use in 54.5% of the patients, which is lower than the previous studies (68%, 88%, and 94.2%).27,30,37 This might be related to the high incidence of glucose-6-phosphate deficiency in our population in which mesalazine is contraindicated.
When patients with moderate to severe CD do not respond to aminosalicylates or immunosuppressive medications, biologic agents like anti-TNF-α monoclonal antibodies are recommended.2 In this study, 22.7% patients used biologic therapy with previous reports of use in 27.8% and 32.2% of patients.28,30 The use of biologic agents in the treatment of patients with CD has significantly increased over the last decade.14 The impact of biologics on the outcome of pediatric CD is clear.6 Besides improving the disease control, they also improve patients’ growth and quality of life.2,6 Biologic therapy appears to be safe and well tolerated by pediatric patients.6 Infliximab, adalimumab, certolizumab pegol, and golimumab are the current four anti-TNF-α agents used to treat patients with CD.14,37,38 The decision to introduce anti-TNF-α agent is based on the disease severity, the presence of comorbidities, direct and indirect costs of medical care, and possible benefits and risks.37 Infections and malignancy are the main potential serious adverse events associated with the use of biologic drugs.2,6 Patients with CD should be closely monitored for neutropenia and leucopenia to avoid life-threating infections especially those on biologics and other immunosuppressive medications.6
We observed that CD was complicated by various adverse events including esophageal strictures, fistula formation, thromboembolic events, and surgical interventions. CD may result in strictures of the affected bowel segments or fistula formation.31 One patient in our study had superior mesenteric artery and vein thrombosis 15 years after the diagnosis of the disease. Patients with IBD are at higher risk of thromboembolic events compared to the general population.39 Mesenteric artery thrombosis due to CD associated coagulation abnormalities is a relatively rare complication of CD.40 In our study, six (11.8%) patients had surgical interventions related to CD out of 51 patients. This is higher than a previous report where surgical interventions were required in seven (7.6%) out of 92 patients with CD (four patients had gut perforations and three had gut strictures).19
Like any other retrospective studies, this study is limited by missing some data related to patients with CD-like history of consanguinity, the use of EEN, and patients’ compliance with medications. In addition, the small sample size of the patients with CD was considered a limitation. However, this study is the first study from Bahrain to shed light on pediatric patients with CD, and it can form a great source of information for any future studies tackling the same group of patients.
Conclusion
There has been a significant increase in both the annual incidence and the prevalence of pediatric CD in Bahrain. We observed a male predominance and high rate of family history of IBD among our cohort, which might indicate a genetic etiology for the disease. The clinical characteristics of the disease in our population are almost comparable to that reported in neighboring countries and worldwide. Abdominal pain and weight loss were the main symptoms at presentation of the disease, and low hemoglobin levels were a common laboratory finding. Terminal ilium is the most frequent area of involvement. Immunosuppressive therapy is the main therapeutic option. Further studies on nutritional status, compliance with medications, and the effect of the disease on the quality of life are needed.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
The authors gratefully acknowledge all those who provide care for patients with CD in the pediatric department, Salmaniya Medical Complex, Kingdom of Bahrain.
references
- 1. Rufo PA, Denson LA, Sylvester FA, Szigethy E, Sathya P, Lu Y, et al. Health supervision in the management of children and adolescents with IBD: NASPGHAN recommendations. J Pediatr Gastroenterol Nutr 2012 Jul;55(1):93-108.
- 2. Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther 2011 Apr;5:185-210.
- 3. Sang L-X, Chang B, Zhang W-L, Wu X-M, Li X-H, Jiang M. Remission induction and maintenance effect of probiotics on ulcerative colitis: a meta-analysis. World J Gastroenterol 2010 Apr;16(15):1908-1915.
- 4. Okou DT, Kugathasan S. Role of genetics in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2014 Oct;20(10):1878-1884.
- 5. Lu Y, Bousvaros A. Immunizations in children with inflammatory bowel disease treated with immunosuppressive therapy. Gastroenterol Hepatol (N Y) 2014 Jun;10(6):355-363.
- 6. Dulai PS, Siegel CA, Dubinsky MC. Balancing and communicating the risks and benefits of biologics in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2013 Dec;19(13):2927-2936.
- 7. Cakir M, Unal F, Dinler G, Baran M, Yuksekkaya HA, Tumgor G, et al. Inflammatory bowel disease in Turkish children. World J Pediatr 2015 Nov;11(4):331-337.
- 8. Sandhu BK, Fell JM, Beattie RM, Mitton SG, Wilson DC, Jenkins H; IBD Working Group of the British Society of Paediatric Gastroenterology, Hepatology, and Nutrition. Guidelines for the management of inflammatory bowel disease in children in the United Kingdom. J Pediatr Gastroenterol Nutr 2010 Feb;50(Suppl 1):S1-S13.
- 9. Docktor MJ, Paster BJ, Abramowicz S, Ingram J, Wang YE, Correll M, et al. Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2012 May;18(5):935-942.
- 10. Rabizadeh S, Dubinsky M. Update in pediatric inflammatory bowel disease. Rheum Dis Clin North Am 2013 Nov;39(4):789-799.
- 11. Al-Mofarreh MA, Al-Mofleh IA. Emerging inflammatory bowel disease in Saudi outpatients: a report of 693 cases. Saudi J Gastroenterol 2013 Jan-Feb;19(1):16-22.
- 12. Srinath AI, Walter C, Newara MC, Szigethy EM. Pain management in patients with inflammatory bowel disease: insights for the clinician. Therap Adv Gastroenterol 2012 Sep;5(5):339-357.
- 13. Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis 2009 May;15(5):778-788.
- 14. Ezri J, Marques-Vidal P, Nydegger A. Impact of disease and treatments on growth and puberty of pediatric patients with inflammatory bowel disease. Digestion 2012;85(4):308-319.
- 15. dos Santos GM, Silva LR, Santana GO; Santos GMd. [Nutritional impact of inflammatory bowel diseases on children and adolescents]. Rev Paul Pediatr 2014 Dec;32(4):403-411.
- 16. IBD working group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). Inflammatory bowel disease in children and adolescents: Recommendations for diagnosis- the Porto criteria. J Pediatr Gastroenterol Nutr 2005;41:1-7.
- 17. Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD, et al; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Colitis Foundation of America. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr 2007 May;44(5):653-674.
- 18. Gasparetto M, Guariso G. Highlights in IBD Epidemiology and Its Natural History in the Paediatric Age. Gastroenterol Res Pract 2013;2013:829040.
- 20. Al-Qabandi WA, Buhamrah EK, Hamadi KA, Al-Osaimi SA, Al-Ruwayeh AA, Madda J. Inflammatory bowel disease in children, an evolving problem in Kuwait. Saudi J Gastroenterol 2011 Sep-Oct;17(5):323-327.
- 21. El Mouzan MI, Abdullah AM, Al Habbal MT. Epidemiology of juvenile-onset inflammatory bowel disease in central Saudi Arabia. J Trop Pediatr 2006 Feb;52(1):69-71.
- 22. El Mouzan M, Saadah O, Al-Saleem K, Al Edreesi M, Hasosah M, Alanazi A, et al. Increasing incidence and prevalence of inflammatory bowel disease in Saudi Arabia: a multicenter national study. Inflamm Bowel Dis 2014;20(6):1085-1090.
- 23. Abdul-Baki H, ElHajj I, El-Zahabi LM, Azar C, Aoun E, Zantout H, et al. Clinical epidemiology of inflammatory bowel disease in Lebanon. Inflamm Bowel Dis 2007 Apr;13(4):475-480.
- 24. Tozun N, Atug O, Imeryuz N, Hamzaoglu HO, Tiftikci A, Parlak E, et al; Members of the Turkish IBD Study Group. Clinical characteristics of inflammatory bowel disease in Turkey: a multicenter epidemiologic survey. J Clin Gastroenterol 2009 Jan;43(1):51-57.
- 25. Ahmaida A, Al-Shaikhi S. Childhood inflammatory bowel disease in Libya: epidemiology and clinical features. Libyan J Med 2009 Jun;4(2):70-74.
- 26. Mehdi A, Baccouche A, Scandrani K. Epidemiology of cytogenetic inflammatory bowel diseases in central east Tunisia. Maghreb Med 1997;314:47-52.
- 27. Ghosh S, Almadi MA. Inflammatory bowel disease: a global disease. Saudi J Gastroenterol 2013 Jan-Feb;19(1):1-2.
- 19. Ludvigsson JF, Büsch K, Olén O, Askling J, Smedby KE, Ekbom A, et al. Prevalence of paediatric inflammatory bowel disease in Sweden: a nationwide population-based register study. BMC Gastroenterol 2017 Jan;17(1):23.
- Aljebreen AM, Alharbi OR, Azzam NA, Almalki AS, Alswat KA, Almadi MA. Clinical epidemiology and phenotypic characteristics of Crohn’s disease in the central region of Saudi Arabia. Saudi J Gastroenterol 2014 May-Jun;20(3):162-169.
- 29. Kim HJ, Kim Y, Cho JM, Oh SH, Kim KM. Therapeutic efficacy of oral enteral nutrition in pediatric Crohn’s disease: a single center non-comparative retrospective study. Yonsei Med J 2016 Sep;57(5):1185-1191.
- 30. Saadah OI, El Mouzan M, Al Mofarreh M, Al Mehaidib A, Al Edreesi M, Hasosah M, et al. Characteristics of pediatric crohn’s disease in Saudi children: a multicenter national study. Gastroenterol Res Pract 2016;2016:7403129.
- 31. Mentzel H-J, Reinsch S, Kurzai M, Stenzel M. Magnetic resonance imaging in children and adolescents with chronic inflammatory bowel disease. World J Gastroenterol 2014 Feb;20(5):1180-1191.
- 32. Mill J, Lawrance IC. Preventing infective complications in inflammatory bowel disease. World J Gastroenterol 2014 Aug;20(29):9691-9698.
- 33. El Mouzan MI, Al Edreesi MH, Al-Hussaini AA, Saadah OI, Al Qourain AA, Al Mofarreh MA, et al. Nutritional status of children with inflammatory bowel disease in Saudi Arabia. World J Gastroenterol 2016 Feb;22(5):1854-1858.
- 34. Chiorean L, Schreiber-Dietrich D, Braden B, Cui XW, Buchhorn R, Chang J-M, et al. Ultrasonographic imaging of inflammatory bowel disease in pediatric patients. World J Gastroenterol 2015 May;21(17):5231-5241.
- 35. Duigenan S, Gee MS. Imaging of pediatric patients with inflammatory bowel disease. AJR Am J Roentgenol 2012 Oct;199(4):907-915.
- 36. Nahidi L, Day AS, Lemberg DA, Leach ST. Paediatric inflammatory bowel disease: A mechanistic approach to investigate exclusive enteral nutrition treatment. Scientifica 2014; Article ID 423817.
- 37. Virta LJ, Kolho K-L. Trends in early outpatient drug therapy in pediatric inflammatory bowel disease in Finland: a nationwide register-based study in 1999-2009. ISRN Gastroenterol 2012;2012:462642.
- 38. Chebli JM, Gaburri PD, Chebli LA, da Rocha Ribeiro TC, Pinto AL, Ambrogini Júnior O, et al. A guide to prepare patients with inflammatory bowel diseases for anti-TNF-a therapy. Med Sci Monit 2014 Mar;20:487-498.
- 39. Papa A, Gerardi V, Marzo M, Felice C, Rapaccini GL, Gasbarrini A. Venous thromboembolism in patients with inflammatory bowel disease: focus on prevention and treatment. World J Gastroenterol 2014 Mar;20(12):3173-3179.
- 40. Sanghavi P, Paramesh A, Dwivedi A, Markova T, Phan T. Mesenteric arterial thrombosis as a complication of Crohn’s disease. Dig Dis Sci 2001 Nov;46(11):2344-2346.