Tuberculosis (TB) has devastated innumerable lives as it has waxed and waned throughout human history. The discovery of effective antituberculosis drugs provided hope to decrease the number of TB associated deaths.1 Unfortunately, despite enormous efforts, TB remains a major cause of morbidity and mortality worldwide. In 2014, 9.6 million people developed TB causing 1.5 million deaths.2
One of the major contributors to TB-related death is drug resistance. The World Health Organization (WHO) declared multi-drug resistant TB (MDR-TB), defined as Mycobacterium tuberculosis resistant to isoniazid and rifampicin, a public health crisis in 2013. Furthermore, the world is witnessing the emergence of extensively drug-resistant TB (XDR-TB), classed as resistance to isoniazid, rifampicin, one fluoroquinolone, and one second-line injectable drug.3
Treatment success rates for drug-sensitive TB and MDR-TB are 85% and 50%, respectively. MDR-TB treatment requires expensive and toxic medications for 20 months or more.2 In addition to being an immense financial burden, drug-resistant TB is associated with a poor outcome. MDR-TB infection has a fatality rate of 12% in non-human immunodeficiency virus (HIV) and 90% in HIV-positive patients.4
Single drug resistance often precedes and predicts the development of MDR-TB. In particular, isoniazid mono-resistance has been associated with high rates of treatment failure and MDR-TB.5 Information regarding any first-line drug-resistant TB in Oman is scarce, with only two other studies performed examining MDR-TB, and their associated clinical factors.6,7 We conducted this study to understand the magnitude and predictors of drug-resistant TB development in Oman. Through this study, we aimed to identify whether drug-resistant TB in our community is due to circulating drug-resistant strains of M. tuberculosis or to inadequate treatment of patients previously treated for TB. This study will aid in the timely management of drug-resistant TB patients and will identify areas for effective resource allocation to control TB.
Methods
We performed a cross-sectional study of patients with M. tuberculosis infection between August 2006 and March 2015, admitted to Sultan Qaboos University Hospital (SQUH), a tertiary care center in Oman. We collected data from all patients who received treatment for TB from the hospital’s electronic medical records: only culture-confirmed cases of M. tuberculosis infections were included in the analysis [Figure 1]. Patients were included only if they had culture-positive M. tuberculosis with available TB drug susceptibility tests results for at least isoniazid and rifampicin. In patients with multiple positive TB cultures, only the first positive culture was included. M. tuberculosis culture and drug susceptibility testing were performed for first-line drugs (isoniazid, rifampicin, ethambutol, and pyrazinamide) using the automated Mycobacterium Growth Indicator Tube (MGIT 960 system; BD Diagnostics, Sparks, MD, USA).8 All cultures were grown at the Central Public Health Laboratory (CPHL), the national TB reference laboratory of the Ministry of Health of Oman.
We adopted the WHO definitions of drug-resistant TB. We defined monoresistant TB as an M. tuberculosis infection resistant to a single antituberculosis drug, MDR-TB as resistant to both isoniazid and rifampicin, and poly-resistant TB as resistant to more than one anti-tuberculosis drug (other than the combination of isoniazid and rifampicin).9,10
To identify the risk factors associated with the development of drug-resistant TB, we compared drug-resistant and drug-sensitive TB cases. Categorical variables were compared using chi-square and Fisher’s exact tests. Wilcoxon rank sum test and Student’s t-test were used to compare continuous variables. A logistic regression model was used to conduct multivariable analysis to compute the odds ratio (OR) between cases of drug-resistant TB and drug-sensitive TB adjusting for confounding covariates.
We used STATA 12 software (StataCorp. 2013, Texas, US) for the data analysis. The Sultan Qaboos University Ethics Committee reviewed and approved the study.
Results
Table 1: Pattern of tuberculosis (TB) drug resistant cases (N = 173) at Sultan Qaboos University Hospital between August 2006 and March 2015.
|
Isoniazid mono-resistant |
3 (1.7) |
|
Rifampicin mono-resistant |
1 (0.6) |
|
Pyrazinamide mono-resistant |
6 (3.5) |
|
Ethambutol mono-resistant |
0 (0) |
From August 2006 to March 2015, there were 260 patients with TB admitted to SQUH. One hundred and ninety (73.1%) patients had culture-confirmed M. tuberculosis infection with isoniazid and rifampicin drug susceptibility testing results documented in 173 (91.1%) patients. Of these, three cases (1.7%) had MDR-TB (resistant to both rifampicin and isoniazid), and 13 cases (7.5%) had drug resistance to a first-line TB drug. Interestingly, all M. tuberculosis isolates were susceptible to ethambutol. Pyrazinamide had the highest mono-drug resistance pattern [Table 1]. TB drug-resistant cases decreased during the study period [Figure 2].
The demographic characteristics of patients with first-line drug-resistant TB are shown in Table 2. In the drug-resistant group, two-thirds of patients were female, and one-third were under the age of 20. Omani nationals had the same risk of drug-resistant TB compared with expatriates. Smoking, alcohol, and drug use were not found to have a significant association with TB drug resistance.
Table 2: Demographic characteristics of patients with culture-positive drug-sensitive and drug-resistant TB.
|
Female |
53 (33.1) |
8 (61.5) |
3.23 |
1.01–10.35 |
0.039 |
|
Age, years |
|
|
|
|
|
|
Mean±SD |
41±19 |
30±21 |
- |
- |
0.068 |
|
Median (IQR) |
36 (25–57) |
26 (17–45) |
- |
- |
- |
|
0–19 |
13 (8.1) |
4 (30.8) |
5.02 |
1.36–18.57 |
0.008 |
|
20–39 |
75 (46.9) |
5 (38.5) |
0.70 |
0.22–2.26 |
0.559 |
|
40–59 |
35 (21.9) |
1 (7.8) |
0.30 |
0.04–2.37 |
0.226 |
|
> 60 |
37 (23.1) |
3 (23.1) |
0.10 |
0.26–3.81 |
0.997 |
|
Omani |
142 (88.8) |
12 (92.3) |
1.52 |
0.19–12.40 |
0.693 |
Data is given as n (%) unless otherwise indicated. SD: standard deviation; IQR: interquartile range; OR: odds ratio; TB: tuberculosis; CI: confidence interval.
Table 3 shows the clinical characteristic of patients with first-line drug-resistant TB. The most striking finding was that one-third of patients who had drug resistance had previous treatment for TB. Only 1 patient (7.7%) had HIV and TB coinfection, and 15.4% had diabetes mellitus (DM). Neither HIV infection nor DM was associated with drug-resistant TB.
Table 3: Univariate analysis of clinical characteristics of patients with culture-positive drug-sensitive and drug-resistant tuberculosis (TB).
|
Previous treatment for TB |
6 (3.8) |
4 (30.8) |
11.41 |
2.72–47.79 |
0.001 |
|
Pulmonary TB |
95 (59.4) |
8 (61.5) |
1.09 |
0.34–3.50 |
0.990 |
|
Extrapulmonary TB |
52 (32.5) |
4 (30.8) |
0.92 |
0.27–3.14 |
0.900 |
|
Disseminated TB |
13 (8.1) |
1 (7.7) |
0.94 |
0.11–7.83 |
0.960 |
|
HIV positive |
11 (6.9) |
1 (7.7) |
1.13 |
0.13–9.50 |
0.910 |
|
DM |
25 (15.6) |
2 (15.4) |
1.02 |
0.21–4.88 |
0.980 |
Data is given as n (%). OR: odds ratio; HIV: human immunodeficiency virus; DM: diabetes mellitus; CI: confidence interval.
Table 4: Adjusted odd ratios (aOR) for risk factors associated with tuberculosis (TB) drug resistance.
|
Gender (female) |
3.85 |
0.039 |
1.07−13.90 |
|
Age < 20 years |
6.80 |
0.009 |
1.61−28.75 |
|
Age > 20 years |
0.08 |
0.006 |
0.01−0.47 |
CI: confidence interval.
Using the WHO classification for the anatomical site of TB infection,11 among drug-sensitive TB cases, we found 59.4% , 32.5%, 8.1% of patients had pulmonary, extrapulmonary and disseminated TB, respectively. We found no significant difference between drug-resistant and drug-sensitive cases by the site of the infection.
Previous treatment for TB was significantly associated with first-line TB drug resistance with an adjusted odds ratio (aOR) 14.81 (95% CI 3.091−70.977, p = 0.001) controlling for gender and age. Female gender was also associated with drug-resistant TB; aOR 3.85 (95% Cl 1.067−13.90, p < 0.039) and age under 20 with aOR 6.80 (95% Cl 1.607−28.75, p < 0.009) [Table 4].

Figure 1: Flow chart of tuberculosis cases diagnosed at Sultan Qaboos University Hospital between August 2006 and March 2005.
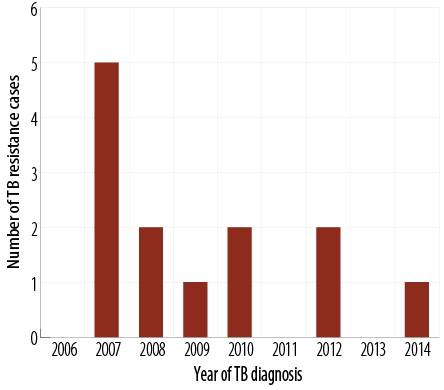
Figure 2: The trend of tuberculosis (TB) drug-resistant cases during the study period.
Discussion
Previous treatment with anti-TB drugs was the strongest predictor for the development of drug-resistant TB. Several other studies have also identified this association.10,12,13 TB drug resistance can occur when anti-TB drugs reach the site of infection in insufficient concentrations. This leads to the selective growth of resistant TB bacterial strains.14 Drug-resistant TB can occur in two contexts. Primary TB drug resistance occurs when a patient becomes infected with a drug-resistant TB strain, while secondary drug resistance occurs when a patient is initially infected with a drug-sensitive strain, and inadequate treatment leads to the appearance of new resistant strain. Our data suggest that the main risk factor for TB drug resistance in our patients was insufficient treatment of previously diagnosed TB.
We estimated the prevalence of MDR-TB within our population to be 1.7%. This is lower than the national average in 2014, which was 2.4%.2 This is also significantly lower than the rates reported in neighboring countries such as the United Arab Emirates, Kuwait, and Saudi Arabia.10,15,16 Of note, in our study, the proportion of any drug-resistant TB was 7.5%, which to our knowledge has never been estimated in Oman.12
We found a statistically significant association between younger age and drug-resistant TB. This is in line with findings reported in previous studies.16−18 This association may be due to the lack of adherence to TB therapy in this age group. An unusual finding in our analysis was the statistical association between female gender and drug resistance. Although a few previous studies demonstrated this finding, the overwhelming majority of studies reported no association between TB drug resistance and gender. One possible explanation for why females have higher risk of resistance is increased exposure as caretakers of sick family members in Omani culture.17−19 We found no significant association between DM, HIV, intravenous drug abuse, and alcohol abuse with TB drug resistance.
A meta-analysis in Europe found a significant association between country of birth and MDR-TB indicating an influence of immigration on MDR-TB rates.20−21 A recent molecular TB study performed in Oman shows evidence of a possible influx of MDR-TB from abroad.7 However, we found no association between nationality and drug-resistant TB. This could be explained by the fact that the most of TB patients seen in our hospital are Omani (88.8% of patients in this study were Omani).
This study had several limitations. Firstly, not all relevant variables associated with TB drug resistance were included. In patients with a history of previous TB, we lacked information about TB culture susceptibility and treatment. However, we were able to assess the magnitude and identify the main risk factors for drug-resistant TB in our patients. Secondly, our study included only patients admitted to a tertiary care center, meaning that the burden of TB drug resistance may be overestimated compared to the national population. Furthermore, we reviewed data over 10-years and found a clinically significant but relatively small number of drug-resistant TB cases, which may have an impact on the accuracy and precision of our results.
Conclusion
The high prevalence of drug-resistant TB found in our study is a public health issue of great concern. Our results show that previous TB treatment, younger age, and female gender were associated with developing drug-resistant TB. Understanding the magnitude and the risk factors of TB drug resistance is a useful adjuvant for clinicians and public health workers involved in the treatment and control of the disease.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Daniel TM. The history of tuberculosis. Respir Med 2006 Nov;100(11):1862-1870.
- 2. World Health Organization. Geneva. Global Tuberculosis Report 2015 [cited 2016 October]. Available from: http://www.who.int/tb/publications/global_report/en/.
- 3. Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, et al. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med 2014 Apr;2(4):321-338.
- 4. Rajbhandary SS, Marks SM, Bock NN. Costs of patients hospitalized for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2004 Aug;8(8):1012-1016.
- 5. Gegia M, Cohen T, Kalandadze I, Vashakidze L, Furin J. Outcomes among tuberculosis patients with isoniazid resistance in Georgia, 2007-2009. Int J Tuberc Lung Dis 2012 Jun;16(6):812-816.
- 6. Mohammadi A, Nassor ZS, Behlim T, Mohammadi E, Govindarajan R, Al Maniri A, et al. Epidemiological and cost analysis of multidrug-resistant tuberculosis in Oman. East Mediterr Health J 2008 Nov-Dec;14(6):1240-1245.
- 7. Al-Maniri A, Singh JP, Al-Rawas O, Al Busaidi S, Al Balushi L, Ahmed I, et al. A snapshot of the biodiversity and clustering of Mycobacterium tuberculosis in Oman using spoligotyping. Int J Tuberc Lung Dis 2010 Aug;14(8):994-1000.
- 8. Adjers-Koskela K, Katila ML. Susceptibility testing with the manual mycobacteria growth indicator tube (MGIT) and the MGIT 960 system provides rapid and reliable verification of multidrug-resistant tuberculosis. J Clin Microbiol 2003;41(3):1235-1239.
- 9. World Health Organization. Geneva. Global Tuberculosis Report 2014 [cited 2016 October]. Available from: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf.
- 10. Munang ML, Kariuki M, Dedicoat M. Isoniazid-resistant tuberculosis in Birmingham, United Kingdom, 1999-2010. QJM 2015 Jan;108(1):19-25.
- 11. World Health Organization. Geneva. Treatment of Tuberculosis: guidelines for national programmes 2009 [cited 2016 October]. Available from: http://www.who.int/tb/publications/tb_treatmentguidelines/en/.
- 12. London: Public Health England, London. Tuberculosis (TB) in the UK: annual report data up to 2013. March 2014 [cited September 2016]. Available from: https://www.gov.uk/government/publications/tuberculosis-tb-in-the-uk.
- 13. Cattamanchi A, Dantes RB, Metcalfe JZ, Jarlsberg LG, Grinsdale J, Kawamura LM, et al. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clin Infect Dis 2009 Jan;48(2):179-185.
- 14. World Health Organization. Geneva. Guidelines for the programmatic management of drug-resistant tuberculosis. 2006 [cited 2016 October]. Available from: http://www.who.int/tb/challenges/mdr/programmatic_guidelines_for_mdrtb/en/.
- 15. Areeshi MY, Bisht SC, Mandal RK, Haque S. Prevalence of TB drug resistance in GCC countries. J Infect Dev Ctries 2014;8(9):1137-1147.
- 16. Granich RM, Oh P, Lewis B, Porco TC, Flood J. Multidrug resistance among persons with tuberculosis in California, 1994-2003. JAMA 2005 Jun;293(22):2732-2739.
- 17. Kurbatova EV, Cavanaugh JS, Dalton T, Click ES, Cegielski JP. Epidemiology of pyrazinamide-resistant tuberculosis in the United States, 1999-2009. Clin Infect Dis 2013 Oct;57(8):1081-1093.
- 18. Espinal MA, Laserson K, Camacho M, Fusheng Z, Kim SJ, Tlali RE, et al. Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int J Tuberc Lung Dis 2001 Oct;5(10):887-893.
- 19. Clark CM, Li J, Driver CR, Munsiff SS .Risk factors for drug-resistant tuberculosis among non-US-born persons in New York City. Int J Tuberc Lung Dis. September 2005;9(9):964-969.
- 20. Murase Y, Maeda S, Yamada H, Ohkado A, Chikamatsu K, Mizuno K, et al. Clonal expansion of multidrug-resistant and extensively drug-resistant tuberculosis, Japan. Emerg Infect Dis 2010 Jun;16(6):948-954.
- 21. Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax 2006 Feb;61(2):158-163.