Histological Changes in the Human Gallbladder Epithelium
associated with Gallstones
Mohamed Zaki, Abdullah Al-Refeidi 2
Zaki M, et al. OMJ. 24, 269-273 (2009); doi:10.5001/omj.2009.55
ABSTRACT
Objectives: Pathological changes related to gallstone formation are still the focus of intensive research. The hypothesis most widely accepted is the stasis of bile caused by gallbladder dyskinesia, while dyskinesia may be the result of pathologic changes in the gallbladder wall. The aim of this study is to investigate the relation between gallstones and light as well as electron microscopic changes in the gallbladder epithelium.
Methods: Gallbladder specimens were collected from patients who underwent cholectstectomy. Paraffin sections were stained with haematoxylin and eosin to demonstrate the general histology. Periodic acid-Schiff and alcian blue were utilized to evaluate the intraepithelial mucin content. Minute specimens were also fixed and processed to evaluate the fine structures of the gall bladder epithelium.
Results: PAS positive materials were increased in the basement membrane. Additionally, an increase in the intensity of alcian blue reaction was observed. At the ultrastructural level, abraded and altered microvilli accompanied by mitochondrial damages, angulated lysosomes, heterogeneous liposomes and damaged organelles were also found. Complete collapse of intercellular spaces in the region above the tight junctions up to the bases of the marginal microvilli was revealed by thin-section electron microscopy. Secondary lysosomes were seen forming complex substructural formations called lipo-mucosomes and collagen fibrils in some cholecystic gallbladders.
Conclusion: Gallstones are accompanied by major changes in the gallbladder epithelium, as shown by both light and electron microscopy. The relation between the observed changes and gallstone formation needs further studies.
From the 1Department of Anatomy, College of Medicine,King Khalid University, Abha, Saudi Arabia, 2Department of Surgery, College of Medicine, King Khalid University, Abha, Saudi Arabia.
Received: 30 Jun 2009
Accepted: 12 Aug 2009
Address correspondence and reprint to: Dr. Mohamed Zaki, Department of Anatomy, College of Medicine, King Khalid University, Abha, Saudi Arabia.
E-mail: mszaki1@hotmail.com
INTRODUCTION
Gallstone disease is a common health problem worldwide including Saudi Arabia.1 It is commonly believed that bile stasis is the prime factor for gallstone formation. The function of the gallbladder is not only to store bile, but also to concentrate it during the interdigestive phase by means of salt-dependent water reabsorption.2 Epithelium of the gallbladder and biliary tract is exposed to high concentrations of potentially harmful exogenous and endogenous compounds excreted into primary bile.3
All columnar epithelial cells are lined by a blanket of mucus, a native physiological gel-like secretion which separates the host mucosal cells from the external milieu.4 The gallbladder mucus plays a regulatory role in cholelithiasis as it promotes the nucleation of stones.5 Mucus, calcium and lipids act in concert to form the gallstones.6 Gallbladder mucin is one of the key factors in gallstone formation. However, there is little information about the diversity of mucin secretion according to the stone composition.7
A major causative agent for stasis is gallbladder dyskinesia which in turn may be a consequence of gallbladder wall pathology.8 However, it was observed that gallbladder tension increased, rather than decreased during the early stage of gallstone formation.9 Cholelithiasis produces diverse histopathological changes in gallbladder mucosa namely acute inflammation, chronic inflammation, glandular hyperplasia, granulomatous inflammation, cholesterosis, dysplasia, and carcinoma.10
The aim of this study is to probe the relation of gallstone and histopathological changes in the gallbladder epithelium
METHODS
Gallbladders of six female patients aged between 27 - 35 years who underwent cholecystectomy for gallstone disease with chronic cholecystitis with multiple stones were obtained. Each gallbladder was sectioned serially from the neck to the fundus. The sections were opened and carefully washed with 0.15 N saline, cut into 2 mm sections and fixed with formol saline for light microscopy and 2.5% gluteraldehyde in O.1M phosphate buffer at pH 7.4 for electron microscopy.
For light microscopy, the formol saline fixed tissues were embedded in paraffin, cut at spam thickness and stained with haematoxylin and eosin (H&E) for studying the general histology, periodic acid-Schiff’s reaction (PAS) and alcian blue for the evaluation of the intra-epithelial mucin content.11
For transmission electron microscopy, one millimeter cube-thick gluteraldehyde-fixed tissue specimen were post fixed with 1% osmium tetroxide in O.1M phosphate buffer, dehydrated in ethanol, cleared in propylene oxide, and embedded in araldite. Semithin sections were prepared using ultramicrotome and stained with toluidine blue then examined with a light microscope. Ultrathin sections exhibiting silver-to-gold colours were stained with uranyl acetate and lead citrate, and examined with JEOL 104 CX electron microscope.12
RESULTS
On light microscopy, the epithelium appeared disrupted with discontinuous, irregular surface and vacuolated cytoplasm (Figs. 1&4). A strong PAS positive reaction was seen in the brush border with many areas of mucous cells of the epithelium (Fig 2) and a deep alcian blue staining reaction in the epithelial surface and the apical part of the columnar cells (Fig. 3).
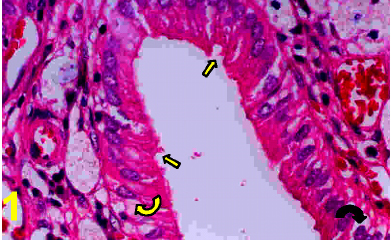
Figure 1: A photomicrograph of a section in a cholecystitic human gallbladder showing disrupted epithelium (arrows) and vacuolated cytoplasm (Curved arrows) with discontinuous epithelium. (H&E X 400)
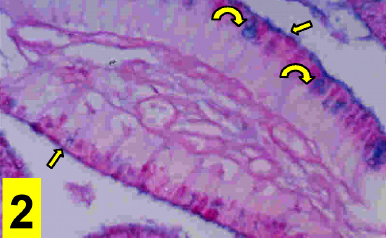
Figure 2: A photomicrograph of a section in the cholecystitic human gallbladder showing a strong PAS positive reaction in the brush border (arrows) and many areas of mucous cells of its epithelium (Curved arrows). (PAS counter-stained with Haematoxylene X400)
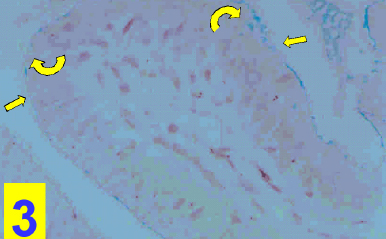
Figure 3: A photomicrograph of a section in the cholecystitic human gallbladder showing a deep alcian blue staining reaction in the the epithelial surface (arrows) and the apical part of the columnar cells (Curved arrows). (Alcian blue stain X400)
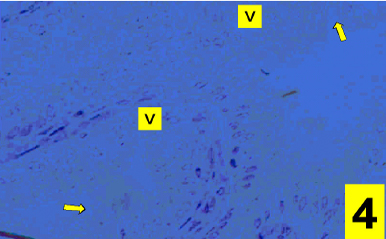
Figure 4: A photomicrograph of a section in the cholecystitic human gallbladder showing a disrupted epithelium and vacuolated cytoplasm (V) and irregular surface of its epithelium (arrows). (Touilidine blue stain X400)
On electron microscopy, columnar epithelial cells that contain apical mucous secretory granules and bulging apices were seen (Fig. 5) and these were actively secreted into the lumen of the gallbladder. The contents of the mucous granules were released by exocytosis to the exterior of the cell (Fig. 6). However, occasionally cells which had their intracellular mucous granules released extracellularly, could be seen with the contents expanded to acquire the form of a spherical mass. In some specimens, cholecystocyte changes were also seen. These are characterized by abraded, altered and spared microvilli with herniation of epithelial cells (Fig. 7) accompanied by mitochondrial damages in its crisatae and heterogeneous lysosomes (Fig. 8). A collapse of intercellular spaces was also observed in these specimens.
Secondary lysosomes containing lipid and lipofuscin deposits, fusions of lipid deposits and mucus-containing vesicles forming complex substructural formations called lipo-mucosomes and collagen fibrils were also seen in some specimens (Fig 9).
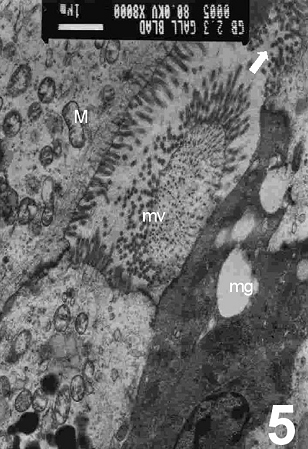
Figure 5: An electron micrograph of the cholecystitic human gallbladder showing the columnar epithelial cells that contain apical mucous secretory granules (mg) and bulging apical apices were seen (arrows). Note shedding of some microvilli (mv) and disrupted mitochondria (M). (X 8000)
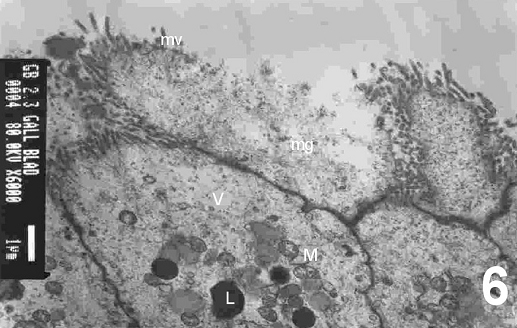
Figure 6: An electron micrograph of the cholecystitic human gallbladder showing mucous granules (mg) with its contents that are released to the exterior of the cell. Note shedding of some microvilli (mv), disrupted mitochondria (M), presence of vacuoles (V) and many lysosomes in the cytoplasm of epithelial cells. (X 6000)
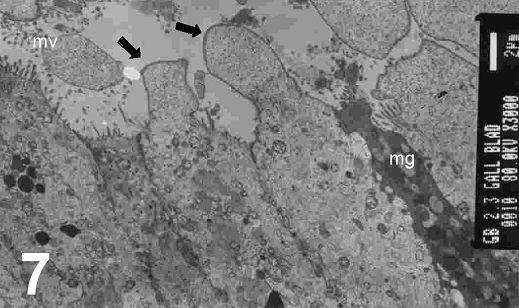
Figure 7: An electron micrograph of the cholecystitic human gallbladder showing abraded and altered and spared microvilli (mv) and herniation of epithelial cells (arrows). Note mucous goblet cells (mg) are incorporated in between the cells (X 3000)

Figure 8: An electron micrograph of the cholecystitic human gallbladder showing mitochondrial damages in its crisatae (M) and heterogeneous lysosomes (L). (X 8000)
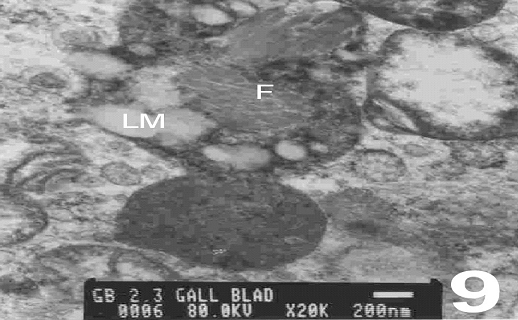
Figure 9: An electron micrograph of the cholecystitic human gallbladder showing secondary lysosomes that contain lipid and mucus-containing vesicles forming complex called lipo-mucosomes (LM) and collagen fibrils (F). (X 20000)
DISCUSSION
Gallstone formation results from many complex factors working together. Among them, the bile stasis caused by impaired gallbladder emptying is thought to be the fundamental kinetic factor.13
The pathologic factors related to gallstone formation are still the hot debate. Bile stasis secondary to gallbladder dyskinesia, is the most widely accepted theory. Gallbladder dyskinesia may be the result of gallbladder wall pathology.8 This view is supported by the finding that sphincterectomy of ampula of Vater may prevent the formation of gallbladder stone and partially improve the contractility of gallbladder.14,15
In the present study, light microscopy examination of sections of the cholecystitic gallbladder, showed disrupted epithelium with discontinuous and irregular surface and vacuolated cytoplasm. The gallbladder epithelium and smooth muscle layer were exposed to concentrated biliary solutes, including cholesterol and potentially toxic hydrophobic bile salts, which are able to influence muscle contraction.16 The surface irregularity was due to the interruption of the brush border which showed a strong PAS positive reaction with abundance of mucous cells in its epithelium. A deep alcian blue staining reaction in the epithelial surface and the apical part of the columnar cells were also prominent in this study. Sulfomucins have a greater role in gallstone formation than the neutral mucins and also that the sialomucins and sulfomucins play an important role in cancer progression and metastasis. The results challenge the glycobiologists to delve deeper in elucidating the role of mucins in gastric malignancy and in gallstone formation. Neutral mucins stained by PAS and sialomucins stained blue by AB at pH 2.5 and sulfomucins are stained brown by AB at pH 1.0.17
Mucin is a high molecular weight glycoprotein that plays an important role in protecting the gallbladder epithelium from the detergent effect of bile.18 Human gallbladder mucin has been implicated to play a role in gallstone disease.19,20
Moreover, it was observed that despite the diverse mechanisms of stone induction and the differences in stone composition, there is a quantitative increase in the epithelial mucus production in the period before stone formation. Specimens were studied by using Priodic acid-Schiff-alcian blue double stain to evaluate the intra-epithelial mucin content. Gallbladder epithelium demonstrates a unique and diverse pattern of mucin core proteins that becomes altered with increasing degrees of inflammation.21
Transmission electron microscopy studies of the cholecystitic gallbladder showed columnar epithelial cells that contain apical mucous secretory granules and bulging apices and these were actively secreted into the lumen of the gallbladder. The contents of the mucous granules were released by exocytosis to the exterior of the cell. However, occasionally cells which had their intracellular mucous granules released extracellularly could be seen with the contents expanded to acquire the form of a spherical mass. A mechanism of mucous granule exocytosis by columnar epithelial cells must take into account the unique physical-chemical properties of mucin glycoproteins and the resultant mucus. Mucins are expressed in a cell and tissue-specific pattern in normal tissue.22 Alterations of the expression pattern of mucins have been described in the formation of gallstones and the expression of neutral mucins was predominant stone-containing gallbladder epithelium.23 In addition, numerous mucous droplets in the apical portion of all the epithelial cells were apparent which confim that the gallbladder epithelium may play an important role in regulating the stone formation.24
Ultrastructural study of a group of selected specimen in the current study revealed cholecystocyte changes characterized by abraded, altered and spared microvilli and herniation of epithelial cells. Mitochondrial damage in the cristae, heterogeneous lysosomes and collapse of intercellular spaces was also observed in these specimens. In one study, abraded and altered microvilli accompanied by mitochondrial damage in the apical regions of the inflamed gallbladders were seen.25 Also, Rokitansky-Aschoff sinuses that are the result of hyperplasia and herniation of epithelial lining of the inflamed gallbladders has been well documented. 26 A complete collapse of intercellular spaces was revealed and the tight junction pathway in the gallbladder does not serve as a route for net fluid transport.27 There is a paucity of information of tight junction proteins in gallbladder epithelium, and disturbances in the structure of these proteins may play a role in the pathogenesis of acute acalculous as well as acute calculous cholecystitis.28 There is a paucity of information of tight junction proteins in gallbladder epithelium, and disturbances in the structure of these proteins may play a role in the pathogenesis of acute acalculous cholecystitis and acute calculous cholecystitis.29
In the present study, secondary lysosomes were seen by electron microscopy, which contained lipid and lipofuscin deposits, fusions of lipid deposits and mucus-containing vesicles forming complex substructures called lipo-mucosomes and collagen fibrils in some specimens. Multivesicular bodies, predominantly in gallbladders with stone were also found.24 Abundant lysosomes and lipid droplets were seen by other workers, therefore, that free cholesterol is absorbed by epithelial cells and thereafter becomes esterified in the endoplasmic reticulum and thus appears as lipid droplets.30 additionally, lipid and lipofuscin deposits, fusions of lipid deposits and mucus-containing vesicles forming complex substructural formations called lipo-mucosomes were demonstrated.31
CONCLUSION
The observations from this study indicate a relationship between pathologic changes of gallbladder epithelium and gallstone formation, and a possible pathway in the pathogenesis of gallstone formation. Overall, the pathological changes of the gallbladder epithelium may play an important role in the process of gallstone formation.
ACKNOWLEDGEMENTS
The authors reported no conflict of interest and no funding was received on this work.
-
Baig SJ, Biswas S, Das S, Basu K and Chattopadhyay G. Histopathological changes in gallbladder mucosa in cholelithiasis: correlation with chemical composition of gallstones. Trop Gastroenterol 2002; 23:25-27.
-
Meyer G, Guizzardi F, Rodighiero S, Manfredi R, Saino S, Sironi C, et al. Ion transport across the gallbladder epithelium. Curr Drug Targets Immune Endocr Metabol Disord 2005; 5:143-151.
-
Aust S, Obrist P, Jaeger W, Klimpfinger M, Tucek G, Wrba F, et al. Thalhammer T Subcellular localization of the ABCG2 transporter in normal and malignant human gallbladder epithelium. Lab Invest 2004; 84:1024-1036
-
Kuver R, Klinkspoor JH, Osborne WR, Lee SP. Mucous granule exocytosis and CFTR expression in gallbladder epithelium. Glycobiology 2000; 10:149-157.
-
Afdhal NH. Choleterol crystal nucleation: A decade- long search for the missing link in gallbladder pathogenesis. Hepatology 1990, 11:669-702.
-
Jacyna MR: Interactions between gallbladder bile and mucosa; Relevance to gallstone formation. Gut 1990, 31:568-570.
-
Kim HJ, Kim JS, Kim KO,Park KH, Yim HJ, Kim JY, et al. [Expression of MUC3, MUC5AC, MUC6 and epidermal growth factor receptor in gallbladder epithelium according to gallstone composition] Korean J Gastroentero. 2003; 42:330-336.
-
Velanovich VF. Biliary dyskinesia and biliary crystals: a prospective study. Am Surg 1997; 63:69-73.
-
Lange K, Gottschalk M. Gallbladder contractility in early stages of lithogenesis in the lithogenic fed guinea pig. Z. Gastroenterol 1995; 33:333-339.
-
Kouroumalis E, Hopwood D, Ross PE, Milne G, Bouchier IA. Gallbladder epithelial acid hydrolases in human cholecystitis. J Pathol 1983; 139:179-191.
-
Bancroft JD, Stevens A. Enzyme Histrochemistry in Theory and Practice of Histological Techniques. 4th ed. Churchill, Livingston, Edinburgh, London, 391-410, 1996.
-
Hayat MA. Principles and Techniques of Electron Microscopy: Biological Application, 3rd ed. Macmillan Press London, 1989.
-
Wei JG, Wang Yao C, Du F, Yu HJ. Dynamic and ultrastructural study of sphincter of Oddi in early-stage cholelithiasis in rabbits with hypercholesterolemia. World J Gastroenterol 2000, 6:102-106.
-
Pitt A, Lillemoe KD. Physiology and pathophysiology of gallbladder motility. Surgical Clin North America 1993; 73: 1267-1290.
-
Li YF, Weisbrodt NW, Moody FG. Effect of bile diversion and sphincterotomy on gallbladder muscle contractility and gallstone formation. Am J Surg 1991 162: 31-35.
-
Portincasa P, Di Ciaula A, VanBerge-Henegouwen GP. Smooth muscle function and dysfunction in gallbladder disease. Curr Gastroenterol Rep 2004; 6:151-62.
-
Ganesh IM, Subramani D, Halagowder D. Mucin glycoarray in gastric and gallbladder epithelia. Journal of Carcinogenesis 2007, 6:10 doi:10.1186/1477-3163-6-10.
-
Vilkin A, Nudelman I, Morgenstern S, Geller A, Bar Dayan Y. Levi Z, Rodionov G, et al. Gallbladder inflammation is associated with increase in mucin expression and pigmented stone formation. Dig Dis Sci 2007; 52:1613-20.
-
Van Wijland MJ, Klinkspoor JH, de Wit LT, Oude Elferink RP, Tytgat GN, Gilloteaux J, et al. Intracellular liposomes and cholesterol deposits in chronic cholecystitis and biliary sludge. Ultrastruct Pathol 2004; 28:123-136.
-
Sheen PC, Lee KT, Liu YE. Mucin Content in Gallbladderswith Brown Pigment Stones or Combination Stones with a Brown Periphery. Digestion 1998 59: 660-664.
-
Ho SB, Shekels LL, Toribara NW, Gipson IK, Kim YS, Purdum, PP, et al. Altered mucin core peptide expression in acute and chronic cholecystitis. Dig Dis Sci 2000; 45:1061-71.
-
Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, Gum ET, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res 1993, 53:641-651.
-
Lee KT, Liu TS. Mucin gene expression in gallbladder epithelium with black pigment stone ascertained by in situ hybridization. The Kaohsiung J Med Sc 2001, 17:517-523.
-
Lee KT, Sheen PC. The ultrastructural study on epithelium of gallbladder with gallstones. Kaohsiung J Med Sci Jan 1996; 12:7-11.
-
Gilloteaux J, Tomasello LM, Elgison DA. Lipid deposits and lipo-mucosomes in human cholecystitis and epithelial metaplasia in chronic cholecystitis. Ultrastruct Pathol 2003; 27:313-21.
-
Cariati A, Cetta F. Rokitansky-Aschoff sinuses of the gallbladder are associated with black pigment gallstone formation: a scanning electron microscopy study. Ultrastruct Pathol 2003; 27:265-70.
-
Frederiksen O, Mollgard K, Rostgaard J. Lack of correlation between transepithelial transport capacity and paracellular pathway ultrastructure in Alcian blue-treated rabbit gallbladders. J Cell Biol 1979; 83: 383-93.
-
Laurila JJ, Karttunen T, Koivukangas V, Laurila PA, Syrjälä H, Saarnio J, et al. Tight junction proteins in gallbladder epithelium: different expression in acute acalculous and calculous cholecystitis. J Histochem Cytochem 2007; 55:567-73.
-
Laurila JJ, Karttunen T, Koivukangas V, Laurila PA, Syrjälä H, Saarnio J, et al. Tight junction proteins in gallbladder epithelium: different expression in acute acalculous and calculous cholecystitis. J Histochem Cytochem 2007; 55:567-73.
-
Satoh H, Koga A. Fine structure of cholesterolosis in the human gallbladder and the mechanism of lipid accumulation. Microsc Res Tech 1997; 39:14-21.
-
Gilloteaux J, Miller D, Morrison RL. Intracellular liposomes and cholesterol deposits in chronic cholecystitis and biliary sludge. Ultrastruct Pathol 2004; 28:123-36.