Epidemiological Study of Cutaneous Adverse Drug Reactions In Oman
ABSTRACT
Introduction: Cutaneous adverse drug reactions (CADR) from all regions of Oman are monitored by spontaneous adverse reaction reporting and monitoring system.
Methods: A total of 100 patients with cutaneous adverse drug reactions were analyzed in a cohort study for 15 months from 1st June 2005 to 31st August 2006.
Results: Out of 100, 85 cases were reported in one year time; from 1st of June 2005 to 31st of May 2006, where 80 were Omani patients and 5 expatriates. Therefore, the incidence was found to be 36 cases per million of total population of Oman (85/ 2,340,815), and 45 per million of Omani population (80/ 1,781,558) for that period. CADR patients comprised 8.5% of the total admitted patients in the skin ward at Al-Nahda Hospital; a tertiary dermatology center. While incidence of CADR among outpatients attending dermatology clinics in Al-Nahda hospital was found to be only 0.3%. Under reporting was the main reason behind this low incidence. However, relatively higher incidence was reported in cases of Toxic Epidermolytic Necrosis (TEN); 2 per million. The clinical patterns and the drugs causing CADR are remarkably similar to those observed in other countries except for minor variations. Urticaria followed by Fixed Drug Eruptions (FDE) and Maculopapular Eruptions (MPE) were the most common reactions. Based on WHO definition of severe ADR; 21% of the cases were classified as severe reactions. One death reported a case of TEN due to Intramuscular Diclofenac. Non-steroidal,anti-inflammatory drugs (NSAIDs) and the antimicrobial agents were the most frequent offending drugs. Most cases of TEN were caused by injection Diclofenac, where most of FDE were caused by NSAIDs.
Conclusion: Based on these results; it was recommended in a circular no. 44/2007/47 from the Director General of the Directorate of Drug Control to restrict the use of Diclofenac Injection.
Key Words
CADR: Cutaneous Adverse Drug Reaction; EM: Erythema Multiforme; FDE: Fixed Drug Eruption; HSSR: Hypersensitivity Syndrome Reaction; MPE: Maculo-Papular Eruption; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; PD: Phoptosensitive Dermatitis; SJS: Stevens Johnson Syndrome; SS: Serum Sickness; TEN: Toxic Epidermal Necrolysis; WHO: World Health Organization.
Submitted: 2 September 2007
Reviewed: 19 November 2007
Accepted: 5 December 2007
From the Department of Dermatology, Al-Nahdha Hospital, Ruwi, Oman.
Address correspondence and reprint request to: Dr. Faiza Al-Raaie, Junior Specialist, Department of Dermatology, Al-Nahdha Hospital, Ruwi, Oman. E-mail: faiza_alraaie@yahoo.com
INTRODUCTION
CADR forms an important clinical entity in dermatology practice, and the severity of such reactions vary from mild to fatal.1 They are among the most frequently reported adverse drug reactions.2
Data regarding the safety profile of a drug prior to marketing is essentially based on preclinical and clinical studies and the later involve only a limited number of subjects. However, when drugs are marketed and used extensively, new adverse events are unearthed. It is estimated that only 50% of the undesirable reactions can be detected during the pre-marketing clinical trials.3
Reporting of adverse drug reaction in the Sultanate of Oman is mandatory as per circular no.2/1994 issued from under secretary office for health affairs at the ministry of Health, Oman. Hence, the Omani National Drug Monitoring Center has been introduced. This center is based in the Directorate of Pharmacy and Drug Control and linked to the World Health Organization’s (WHO) drug-monitoring programme in Uppsala (Sweden) since 1995. The programme functions on the basis of national pharmacovigilance centers coordinated by the WHO programme for international drug monitoring.
Prior to this study, however, there were no published data that evaluate those reported CADR in Oman; the incidence, the clinical spectrum, and the offending drugs. Hence this study provides an interesting and relevant topic for a detailed study.
The aim of this study is to assess the incidence of CADR in Oman, and the incidence of hospitalized patients as well as outpatients in Al-Nahda Hospital with CADR in a year time. Furthermore, to also identify the clinical spectrum of CADR in Oman, the characteristics of patients with those reactions, the offending drugs, and establish a causal link between the drug and the reaction by using World Health Organization (WHO) causality definitions.
METHODS
Letters explaining the aims of study and emphasizing reporting to the ministry through the confidential ADR form were faxed from the dermatology department in Al-Nahda Hospital to all dermatologists in different areas of Oman prior to June 2005.The ministry confidential ADR form requires reporting of general information like sex and age of the patient. It also requires reporting information about the suspected drug; its route, daily dose, date started and date stopped the indication, and other drugs a patient is on. In addition, information about the suspected reaction, date of onset, date stopped, and the outcome of the reaction. It also requires general information about the treating physician/ doctor; his/her phone number, name and location of the health center. Further information about past medical history and atopic background for every patient was also obtained.
New reports of the cutaneous reactions from all regions of the sultanate were collected every week from Omani National Drug Monitoring Center, since 1st of June 2005.
All the patients with CADR from Muscat region were further interviewed and examined clinically. Because of logistic reasons; information required about the rest of patients were completed through phone calls.
DIAGNOSIS
The WHO definition of ADR was used; “any noxious, unintended, and undesired effect of a drug, which occurs at doses used in humans for prophylaxis, diagnosis, or therapy”.4
The diagnosis of drug reaction was basically clinical. Only for uncertain cases, further in vivo and in vitro tests were required. In vivo tests included dechallenge and rechallenge of the offending drug. In vitro tests included skin prick test and skin biopsy.
Dechallenge of the offending drugs was done in all patients. However, Rechallenge of the offending drugs was done only for selected cases. Standard skin prick test was not available; however, it was substituted by diluted intradermal test dose for selected cases. Both skin prick test and rechallenge test were avoided in patients with severe drug reaction, or in cases which have the potential to progress into severe reactions such as urticaria and erythema multiforme (EM). It was also avoided in paediatric and elderly age groups, and in sick patients. Skin biopsy was done only in specific cases to confirm drug reaction, when differentiation between the idiopathic from the drug-induced reaction was needed. All these tests were done before including the patients in this study, not only to confirm diagnosis, but also to classify the drug reaction into the proper causality definition; certain or probable.
Cases of drug reaction were excluded when the drug was topical, the drug history was not clear, and the drug reaction was unlikely or unclassified according to the WHO causality definition.
LIMITATIONS
The basic problem faced in this study was under-reporting of cases of drug reaction which unfortunately was due to lack of compliance in the part of some dermatologists and physicians, which also had its impact in underestimating the true incidence. According to Mrs. Madiha AMaskari; Section Head in Omani National Drug Monitoring Center; the center has tried to solve the problem of under-reporting by conducting three workshops in the sultanate that covered all regions to try to stimulate reporting from health care providers. In order to detect factors of under-reporting in the sultanate, a questionnaire was distributed during the three workshops. The questionnaire results concluded that 40% of the participants do not report drug reactions, due to the following reasons: lack of knowledge about the system, unavailability of forms, lack of knowledge of filling out the forms, lack of time, or lack of confidentiality for the reported information.
Moreover, improper reporting for cases reported from the peripheral areas; as completion of the questionnaires was only through phone calls. However, cases were included only if they were examined by two physicians/general practitioners.
RESULTS
A total of 100 patients with CADR were included in this study. Out of them; 95 patients were Omani patients, while only 5 patients were expatriates. 76 patients were outpatients at the time of developing the drug reaction, while 24 patients were inpatients in different departments from different hospitals.
Incidence
Out of 100, 85 cases were reported in one year time; from 1st of June 2005 to 31st of May 2006. 80 were Omani patients and 5 expatriates. The incidence was found to be 36 cases per million per year among total population of Oman (85/2,340,815), and 45 per million per year among Omani population (80/1,781,558) for that period.
For the same period; 8.5% of total admission in the skin ward in Al-Nahda Hospital was for patients with purely CADR (25/295). While incidence of CADR among outpatients in Al-Nahda hospital was about 0.3% of total outpatients (28/ 8812).
Sex Ratio
52 male patients and 48 patients were involved in the study with approximately equal male to female ratio (1:0.92).
Age-wise Distribution
The mean age group was 32 (range 1 to 70 years). Figure 1 shows age groups and sex distribution of patients.
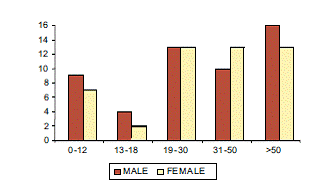
Figure 1: Age groups and sex distribution of patients
Regional Distribution
Most of the cases were reported from Muscat. Cases from other regions are listed in Table 1.
Table 1: CADR cases among Regional Population
Region |
Patients |
Pop-2005 5 |
Muscat |
46 |
695,432 |
Al Batinah |
22 |
688,172 |
Al Sharqiyah |
16 |
330,860 |
Al Dakhliyah |
8 |
280,687 |
Dhofar |
4 |
234,709 |
Al Dhahirah |
2 |
223,473 |
Musandam |
2 |
30,637 |
Al Wusta |
0 |
24,867 |
8 regions total= 2,508,837 |
|
|
Past Medical History
Figure 2 presents past medical history of patients.
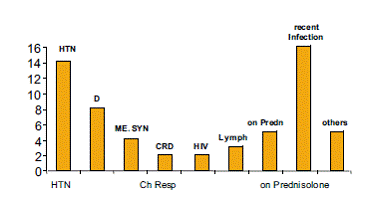
Figure 2: Past Medical History
Pre-Existing Atopy
Twenty one patients had personal and/or familial background of atopy (dermatitis, rhinitis, conjunctivitis and/or bronchial asthma). While 79 patients denied to have history of atopy. History of atopy was present in 44% of cases of urticarial and morbiliform rashes which is found to be significant (Pearson Chi-Square= 0.001).
Clinical Spectrum of Cadr
Table 2 represents the clinical types of CADR reported.
Table 2: Types of Cutaneous Adverse Drug Reactions
Drug Reaction |
Frequency |
Urticaria |
35 |
Fixed Drug Eruption |
14 |
Maculopapular |
13 |
Angiodema |
6 |
Erythema Multiforme |
6 |
Steven-Jonson Syndrome |
5 |
Acniform / Follicular eruption |
5 |
Toxic Epidermolytic Necrosis |
4 |
Exfoliative Dermatitis |
4 |
Photosensitivy drug eruption |
2 |
Hypersensitivity syndrome |
1 |
Serum Sickness |
1 |
other Bullous drug reaction |
1 |
Vasculiis |
1 |
Others |
2 |
Causative Drugs
NSAIDs and the antimicrobial agents were the most frequent offending drugs, each were responsible in 29 patients. NSAIDs included Mefenamic acid, Diclofenac, Ibuprofen, Meloxicam and Tenoxicam. Antimicrobial agents include penicillins in 18 patients, cephalosporins in 3 patients, sulpha-group drugs in 4 patients, Erythromycin in two patients, and each of Metronidazole, Rifampicin, and Piperacillin in one patient. Table 3 represents the causative drug groups.
Table 3: Causative Drugs
Involved Drug |
Frequency |
NSAIDs |
29 |
Pencillins |
18 |
Antiepileptics |
8 |
Sulfonamides |
4 |
Steroids |
4 |
Cephalosporins |
3 |
ACE Inhibitors |
3 |
Diuretics |
3 |
Allopurinol |
3 |
other Antibiotics |
3 |
Antihistamines |
2 |
Antineoplastic |
2 |
B-blockers |
1 |
Aspirin |
1 |
Antimalarials |
1 |
Antituberculous |
1 |
Others |
14 |
NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; ACE: Angiotensin Converting Enzyme |
|
Severity & Death
Based on WHO definition of severe ADR; 4 21 cases were classified as severe reactions. These cases are SJS, TEN, HSSR, SS, and ED.
Only one death reported from an interior hospital in a 45 years old expatriate female patient who developed TEN after receiving injection diclofenac and went into serious complications.
Causality Classification
Based on WHO causality definition; 56 of cases are classified as certain drug reactions; 43 as probable, and only one case was debated then finally classified as possible.
DISCUSSION
One step we took to reduce heterogeneity was to exclude any data that did not fit the WHO definition of ADR. This definition excludes therapeutic failures, intentional and accidental poisoning (i.e., overdose), and drug abuse. Also, this does not include adverse events due to errors in drug administration or noncompliance (taking more or less of a drug than the prescribed amount). Using this conservative definition avoids overestimating the CADR incidence.
An accurate estimate of the incidence of CADR is difficult to achieve, despite attempts at monitoring by the government. One problem is the lack of standardized coding for drug reactions. Moreover, the information that is available must be interpreted with considerable care, because data will be biased, depending on the method of collection. Thus, data on medical inpatients, especially from acute care facilities, may indicate a relatively high incidence, since these patients are generally sicker and receive more intensive drug treatment. By contrast, spontaneous reporting may underestimate the true incidence.6
Most of studies done in other countries have estimated the incidence of CADR among inpatients in a tertiary hospital.1, 7-11 About 8.5% of total admissions in the skin ward in Al-Nahda Hospital, in one year time, was due to cases of CADR; (25/295). This is close to the incidence reported in other studies; where about 3-8% of hospital admissions are consequences of adverse drug reactions.6, 12 These admitted cases are not necessarily severe reaction, but they are moderate to severe cases which need close management.
Estimating the incidence in referring to the total population per year is a well known method used by different references for different skin diseases.6, 14 By using this method, many studies have estimated the incidence of special form of drug reaction; TEN is the most common example. In our study, the incidence of CADR per population per year has been estimated. However, comparison to other studies is possible only for cases of TEN.
Elderly and adult age groups were the main age groups affected. Patients from these age groups are probably more exposed to drugs. Similar observation was reported in a previous study.15 In another study; patients aged 20-49 years were at greatest risk of drug eruptions, probably due to increased exposure to antibiotics.16 The difference in various studies may be related to the regional variation in the health care seeking behaviour of the population.17
The regional distribution of reported cases is parallel to population density in these areas as shown in the Table 1.
CADR vary in their patterns of morphology and distribution. In this study, the most common morphologic patterns were Urticaria followed by FDE and MPE. Same finding was reported from recent studies done in Kuwait, 18 and in North India.19 While MP was the most common one in another study.17 This variation could be due to different patterns of drug usage and different ethnic group characteristics.18
The course and outcome of drug-induced disease are also influenced by host factors. Patients with previous history of a drug reaction are more likely to develop reactions from other drugs.19 In this study; 18% of the patients gave history of previous drug reaction.
Active viral infection, underlying disease and concurrent medications have been shown to alter frequency of drug-associated eruptions. In addition, the patient’s immune status and clinical condition may influence the rate of adverse reactions. As shown in Figure 2; only 2% of patients were HIV patients. This is actually much lower than what was reported in other studies, 18, 20 which emphasis the fact that HIV is a risk factor to develop ADR. It is possible that underreporting is a factor for this low incidence. However even in daily clinical practice we don’t frequently see HIV with cutaneous drug reactions. Most of our HIV patients, because of cultural aspect, do not follow up, and they seek for treatment abroad.
Atopy background was present in 44% of cases of urticarial and morbiliform rashes which could be considered as a predisposing factor for the reactions as it is found to be significant (Pearson Chi-Square= 0.001). Previous studies also have emphasized the role of atopy in drug allergy.19, 21 In a study puplished in European Journal of Allergy & Clinical Immunology; the prevalence of atopy is increased in challenge-proven NSAID-intolerant patients.21
NSAIDs and the antimicrobial agents were the most frequent offending drugs in this study with equal proportion of patients affected, followed by antiepileptic drugs. In most of other studies, antimicrobials represented the major causative group, followed by antiepileptic drugs, and NSAIDs. 17, 18, 20
Most cases of TEN reported in this study were caused by the injection Diclofenac, and most cases of FDE were caused by NSAIDs. While the main causative drugs in most other studies are the antimicrobial agents such as sulphonamides and penicillin.17, 18, 20, 22 Hence, there may be pharmacogenetic predispositions among Omani population to react against NSAIDs in general, and against injectional diclofenac in particular. NSAIDs were as important as antimicrobial agents in one study from Finanld.23
Despite under-reporting; incidence of TEN in this study is found to be 2 per million (total 4 patients and 5 episodes); which just higher than incidence reported in other studies (0.5 to 1.2 per million).24, 25, 26 Most of TEN cases were caused by Intramuscular Diclofenac. Death reported in one patient. TEN was the only cause of death in another Indian study.27
Based on these results; it was recommended in a circular no. 44/2007/47 from the Director General of the Directorate of Drug Control to restrict Diclofenac Injection.
Sulfonamides in this study were responsible for 4 cases. This is much lower than what was reported in other studies.17, 18, 22 This is may be partially because these drugs are avoided most of the time unless really required, as G6PD deficiency is common in Oman.
Despite acneiform eruptions is expected in patients on regular steroid, we wanted to highlight the fact that prednisolone was found to be the most common cause of drug-induced acne(3 out of 5 cases), followed by antiepileptic drugs (2 cases).
Antihistamines are a group of drugs that are used very frequently in daily clinical practice. Once a drug reaction is suspected, the attention is brought to the common drugs that cause drug reactions such as antibiotics; one may neglect other less common drugs such as antihistamines. This study shows that 2% of cases were caused by Cetrizine and Chlorpheniramine maleate. This is emphasizing the importance of reporting drug reaction. Only by reporting drug reaction we will know what the common offending drugs are.
A comparison of the clinical types of drug eruption observed in this study with those found in some of other studies is shown in table 4.
Table 4: Comparison of Types of Cutaneous Adverse Drug Reactions
Clinical type |
Our study (Oman) |
F.Ghanem&N. Mutairi (Kuwait) 18 |
Sharma& Dhar (India) 17 |
Stubb at al (Finland)23 |
Alanko et al (Finland) 28 |
Pauvilai & Choonhakarn (Bangkok) 22 |
MPE |
13% |
38% |
26% |
39% |
32% |
60% |
Urticaria & Angiodema |
41% |
23% |
6% |
18% |
20% |
6% |
FDE |
14% |
14% |
22% |
39% |
34% |
9% |
SJS,TEN |
9% |
8% |
22% |
|
1% |
8% |
EM |
6% |
6% |
20% |
|
2% |
4% |
Exf. Dermatitis |
4% |
3% |
4% |
|
1% |
4% |
Other Bullous DE |
1% |
2% |
|
|
|
|
Photoallergic DE |
2% |
1% |
|
|
|
1.5% |
Vasculitis |
1% |
|
|
|
|
2% |
Others |
9% |
3% |
|
4% |
11% |
5% |
MPE: Maculo-Papular Eruption; FDE: Fixed Drug Eruption; SJS: Steven Johnson Syndrome; TEN: Toxic Epidermal Necrolysis; EM: Erythema Multiforme; DE: Drug Eruption |
||||||
A comparison of the causative agents of drug eruption found in this study and some of other studies is shown in Table 5.
Table 5: Comparison of the Causative Drugs
Offending drug class |
Our study (Oman) |
F. Ghanem & N. Mutairi (Kuwait) 18 |
Sharma & Dhar (India) 17 |
Stubb et al. (Finland)23 |
Alanko et al. (Finland) 28 |
Pauvilai & Choonhakarn (Bangkok) 22 |
Antimicrobials |
29% |
43% |
54% |
39% |
42% |
60% |
NSAIDs |
29% |
8% |
|
35% |
27% |
10% |
Antiepileptics |
8% |
19% |
34% |
18% |
10% |
8% |
Others |
34% |
29% |
12% |
9% |
20% |
22% |
NSAIDs: Non-Steroidal Anti-Inflamatory Drugs |
||||||
The mean reaction time was found to be 10 days. Most patients (94) developed the drug reaction within 6 weeks of taking the 1st dose of the drug. Most of them developed the rash while taking the incriminated drug. According to references; 4 the reaction time of the offending drugs is plausible.
Oral provocation is rechallenging the suspected drug. It is still the only reliable clinical method for identifying the causative agent. The procedure involves only a minimal risk when performed rationally and with caution. Stubb et. al23 concluded that verifying the drug responsible for the eruption is of paramount importance, and oral provocation is the proper method for detecting the causative agent. It is better to induce a mild reaction under controlled circumstances than to allow the patient to suffer repeated severe reactions at home. Positive rechallenge makes the drug reaction certain. Attributing causality as per the WHO causality definition, 56% of cases were classified as certain drug reactions; 43% as probable, and only 1% of the cases was classified as possible. These results add reliability to this study and reduce false positive cases.
It may be concluded that the clinical patterns and the drugs causing ADR are remarkably similar to those observed in other countries except for minor variations. This study is a message to health care providers especially physicians and dermatologists reminding them about the importance of reporting every drug reaction they face, and re-emphasizing the utility of an efficient pharmacovigilance system that could generate valuable data about drug safety for health care deliverers and their beneficiaries in our country.
ACKNOWLEDGMENT
This work was granted by God, and done only with cooperation of Pharmacist Madiha Al-Maskari; Section Head, and Dr. Shirly S. V; Senior Pharmacologist, in Omani National Drug Monitoring Center, under Directorate of Drug Control. The authors also like to express sincere appreciation for late Dr Abdul Raouf Al-Suwaid; Senior Consultant and Head of Dermatology and Genito-Urinary Medicine for his kind encouragement and continuous guidance.
REFERENCES
-
Bigby M. Rates of cutaneous reactions to drugs. Arch Dermatol 2001; 137:765.
-
Arndt K A, Jick H. Rates of cutaneous reactions to drugs: A report from the Boston Collaborative Drug surveillance Program. JAMA 1976; 235:918-922.
-
Edwards R, Aronson JK. Adverse drug reactions: Definitions, diagnosis and management. Lancet 2000; 356:1255-9. BMJ 2004;329:15-19.
-
Requirements for adverse drug reaction reporting. Geneva; World Health Organization, 1975.
-
Statistical Year Book 2006. Sultanate of Oman, Ministry of National Economy.
-
S. M. Breathnach. Drug Reaction. In: Rook et al. Text Book of Dermatology. 7th Edition. London, 2004. p. 73.1- 73.180, 74.1- 74.20.
-
Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994; 331:1272-1285.
-
Craig KS, Edward WC, Anthony AG. Cutaneous drug reactions. Pharmacol Reviews 2001; 53:357-379.
-
Noel MV, Sushma M, Guido S. Cutaneous adverse drug reactions in hospitalized patients in a tertiary care centre. Indian J Pharmacol 2004; 36:292-5.
-
Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a South Indian hospital-their severity and cost involved. Pharmacoepidemiology and Drug Safety 2003; 12:687-692.
-
Pudukadan D, Thappa DM. Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India. Indian J Dermatol Venereal Leprol 2004; 70:20-24.
-
Levy M, Kewitz H, Altwein W et al. Hospital admissions due to adverse drug reactions: a comparative study from Jerusalem and Berlin. Eur J Clin Pharmacol 1980; 17:25-31.
-
Chatterjee S, Ghosh AP, Barbhuiya J, Dey SK. Adverse cutaneous drug reactions: A one year survey. Department of Pharmacology, Institute of Postgraduate Medical Education and Research, 244 B, Kolkata, India
-
W. Sterry, R. Paus, W. Urgdorf. Drug reaction Thieme Clinical Companions Dermatology. 5th Edition. Germany, 2005. p. 179-189.
-
Hafner JW, Belknap SW, Squillante MD, Bucheit KA. Adverse drug events in emergency department patients. Ann Emerg Med 2002; 39:258-267.
-
Solensky R, Mendelson LM. Systemic reactions to antibiotics. Immunol Allergy Clin N Am 2001; 21:679-697.
-
Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: Clinical pattern and causative agents-A six-year series from Chandigarh, India. J 2001; 47:95-99.
-
Faisal Al-Ghanem, N. Al-Mutairi, Sepctrum of Cutaneous Adverse Drug Reactions in the Emergency Department of Al-Farwaniya Hospital. The Middle East Journal of Emergency Medicine; Kuwait, Sept 2006
-
Odom T. B., James W. D., Berger T. G. Contact Dermatitis and Drug eruptions. Andrew’s et al. Diseases of the Skin. Philadelphia 2006 10th Edition. Pages 95-145.
-
Fiszenson-Albala F, Auzerie V, Mahe E, Farinotti R, Durand-Stocco C, Crickx B, Descamps V. Dep. Of Derm. Hospital Bichat-Claude Bernard, Paris, France.
-
Pastorello, E. A. 1; Zara, C. 1; Riario-Sforza, G. G. 2; Pravettoni, V. 1; Incorvaia, C. 1Atopy and intolerance of antimicrobial drugs increase the risk of reactions to acetaminophen and nimesulide in patients allergic to nonsteroidal anti-inflammatory drugs. European Journal of Allergy & Clinical Immunology 53(9):880-884, September 1998.
-
Puavilai S, Choonhakarn C. Drug eruptions in Bangkok: a 1-year study at Ramathibodi Hosp. Int J Dermatol 1998; 37: 747-751.
-
Stubb S, Heikkila H, Kauppinen K. Cutaneous reactions to drugs. A series of in patients during a five-year period. Acta Dermatol Venereal (Stockb) 1994; 74: 289- 291. University Central Hospital Helsinki, Finland.
-
Chan H-L, Stern RS, Arndt KA et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol 1990;126: 43-7.
-
Roujeau J-C, Guillaume J-C, Fabre J-D et al. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981-1985. Arch Dermatol 1990; 126: 37-42.
-
Li LF, Ma C. Peking University, Third Hospital, Dept Dematol, 49 China. Epidemiological study of severe cutaneous adverse drug re-doi.wiley.com/ 10.1002/pds.1368
-
Jhaj R, Uppal R, Malhotra S, Bhargava VK. Cutaneous adverse reactions in in-patients in a tertiary care hospital.Indian J Dermatol Venereol Leprol 1999; 65:14-7.
-
Alanko K, Stubb S, Kauppinen K. Cutaneous drug reactions: clinical types and causative agents. A five-year survey of in patients (1981-1985). Acta Dermatol Venereal (Stockb) 1989; 69: 223-226. University Central Hospital Helsinki, Finland.