Sleep quality and duration are well recognized for their significance in regulating and optimizing various metabolic and physiological functions in the human body. Studies have shown that poor sleep quality and inadequate sleep duration are linked to adverse health effects on both physical and mental well-being.1 The National Sleep Foundation recommends that adults get 7–9 hrs of sleep per night for optimal health.1,2 Factors such as extended working hours, cultural and religious practices, environmental influences, seasonal variations, and emerging communication technologies can all significantly affect sleep quality, duration,
and patterns.1,3
The association between poor sleep quality and duration with cardiovascular disease (CVD) risk is supported by an expanding body of evidence.4–7 Hypertension, type 2 diabetes mellitus (T2DM), an atherogenic lipid profile, and obesity—all well-known risk factors for CVD—are closely associated with sleep quality and duration.1,4,8,9 Both short (< 7 hrs per night) and long (> 10 hrs per night) sleep durations, as well as daytime napping, have been linked to an increased risk of CVD in large-scale prospective cohort studies and meta-analyses.4–7 Data from the Sleep Heart Health Study demonstrated that sleep efficiency, the ratio of total time spent sleeping to time spent in bed, was significantly correlated with increased CVD risk and CVD-related mortality when sleep efficiency was below 80%.5
A 2023 study conducted in the United States found significant associations between sleep duration and disturbances and various cardiovascular biomarkers, such as high-density lipoprotein, low-density lipoprotein, triglycerides, C-reactive protein, glycated hemoglobin, insulin, and blood glucose. The findings indicated that both short and long sleep durations, as well as sleep disturbances, were linked to abnormal levels of these biomarkers, suggesting an increased risk of CVD.7 Additionally, a meta-analysis of 249 324 adults reported a 50% increased risk of developing coronary heart disease among those sleeping < 7 hrs per night. The same study also associated long sleep duration (> 10 hrs) with an increased risk of coronary heart disease, stroke, and overall CVD risk.4
Sleep duration and quality are direct components of sleep patterns, with the internal circadian rhythm regulating specific sleep timing and ensuring sufficient duration for daytime alertness.3 An individual’s sleep pattern is defined as the clock-hour plan of sleep time and wake times, including nap habits and any disruptions to sleep.10 There is growing evidence that not only sleep duration but also sleep patterns adopted in one’s lifestyle can influence health outcomes.9
Two research studies conducted in Oman explored the relationship between sleep patterns and metabolic abnormalities.9,11 The first study found that napping for over an hour in the afternoon was significantly associated with abnormal glycated hemoglobin and body mass index (BMI), increasing the risk of developing T2DM.9 The second study reported that regular sleep deprivation was significantly linked to T2DM, irrespective of age, sex, BMI, or family history of diabetes.11 Both studies underscored the need for public health interventions promoting healthy
sleep habits.9,11
Sleep patterns are generally categorized into three types: monophasic, biphasic, and polyphasic.12 Monophasic sleep consists of a single nighttime sleep period; biphasic sleep includes a longer nighttime sleep and a short daytime nap; and polyphasic sleep involves multiple sleep periods throughout the day.12 Arab Muslims often exhibit distinct sleep patterns due to five daily prayers, with the first at dawn, thereby dividing nighttime sleep into two segments.13 Furthermore, a recent study conducted in Oman identified four distinct sleep patterns: monophasic, biphasic (post-dawn), biphasic (afternoon siesta), and polyphasic.3 The study found that the most prevalent sleep pattern among participants was biphasic (afternoon siesta), followed by polyphasic, monophasic, and biphasic (post-dawn).3 There is a lack of research investigating the association between sleep patterns, sleep quality, and CVD risk in some areas of the world, particularly in Muslim Middle Eastern societies, such as Oman, where habitual sleep behavior differs significantly from that in Western societies. While numerous studies have suggested an association between sleep quality, sleep duration, and CVD risk, few have explored this relationship within Arab Muslim societies, considering the distinct sleep patterns typically adopted in this region.
The objective of this study was to explore the potential relationship between sleep quality among Omani adults attending primary healthcare centers in Muscat and their 10-year risk of developing CVD. Additionally, the study aimed to assess sleep quality and patterns, alongside the 10-year CVD risk in the same population. The relationship between various sleep patterns—monophasic, biphasic (bi-dawn and siesta), and polyphasic—and cardiovascular risk is investigated, as well as the effects of work schedules and job nature on cardiovascular health and their impact on sleep quality. Furthermore, the research examines the influence of sociodemographic factors, such as education level, on sleep quality and CVD risk. Another important focus is on the association between BMI and sleep quality, and the potential impact of BMI on the risk of developing CVD.
Methods
A cross-sectional design was conducted to examine the relationship between sleep quality, sleep patterns, and the 10-year risk of developing CVD among Omani adults attending primary healthcare centers in Muscat. Conducted over 12 months, from September 2023 to September 2024. The data was collected at three selected healthcare centers: Al-Azaiba, Shadi, and Seeb, chosen for their representativeness, accessibility to participants, and high-quality facilities. The study targeted Omani adults aged 30–75 years, as this age range is specifically relevant for cardiovascular risk assessment using the Framingham Risk Calculator. To ensure reliability and generalizability, the initial sample size of 331 was calculated based on the prevalence of moderate to high cardiovascular risk in the Omani population. To enhance the robustness of the findings, the sample size was increased to
400 participants.
A simple random sampling technique was applied to select participants, minimizing selection bias and ensuring that every eligible individual had an equal chance of being included. Individuals with established cardiovascular disease, non-Omani, those outside the specified age range, and those presenting with signs of obstructive sleep apnea (as indicated by the STOP-Bang questionnaire) were excluded.
Trained staff nurses collected the data through direct interviews in the triage clinics of the selected healthcare centers. Participants’ 10-year cardiovascular risk was estimated using the non-laboratory Framingham Risk Score (FRS), which incorporates variables such as age, history of diabetes, smoking status, systolic blood pressure, and BMI. Sleep quality and patterns were assessed using the Pittsburgh Sleep Quality Index (PSQI), a validated instrument that evaluates various dimensions of sleep over one month. A score of 5 or higher indicated poor sleep quality. Additional demographic information and physical activity levels were also collected.
Data was analyzed using SPSS (IBM Corp. Released 2023. IBM SPSS Statistics for Windows, Version 29.0.2.0 Armonk, NY: IBM Corp.). Descriptive statistics, t-tests, analysis of variance, and chi-square tests employed to identify significant associations between variables. Data validity and reliability were ensured by utilizing standardized instruments, such as the FRS and PSQI, and by providing thorough training to data collectors.
Ethical approval was obtained from the Ministry of Health, Oman (MoH/CSR/23/27053), and informed consent was obtained from all participants. For illiterate participants, the consent form and questionnaires were read aloud to ensure comprehension. Participants were assured of the confidentiality of their responses and the voluntary nature of their participation, with no impact on the healthcare services they received.
Results
Our study analyzed data from 400 individuals across three healthcare centers: Shadi (33.0%), Al-Azaiba (29.8%), and Seeb (37.3%). Most (60.0%) participants were female, non-smokers (85.5%), and married (82.5%). The majority (81.5%) of participants did not have diabetes, 75.3% were not undergoing hypertension treatment, and 65.8% had no comorbid conditions. Educational status among the participants varied with 28.0% having a college or university-level education and 17.8% being illiterate. Occupationally, participants were almost equally split among office-based work (33.5%), field-based work (34.0%), and non-working (32.5%). Almost half (44.8%) worked regular fixed office hours, while 34.3% had flexible work timings and 21.0% worked shifts, reflecting diverse working conditions. In terms of age, 60.0% were aged 30–44 years, 34.0% were aged 45–64 years old, and 6.0% were aged 65–75 years old. The mean BMI was 29.1 ± 6.4, indicating an overweight population, with 34.3% categorized as overweight and 35.3% as obese. The average systolic blood pressure was 126.9 ± 14.0 mmHg [Table 1].
-web-resources/image/OS-OMJ-D-25-00262_Table-1.png)
 Table 1
Table 1
Sleep quality, as indicated by the PSQI [Table 2], was reported as good by 60.0% of participants, with an average sleep duration of 6.8 ± 1.5 hrs per night. The mean bedtime was 10:30 PM and wake-up time was 6:30 AM. Most (59.5%) participants follow a biphasic (afternoon siesta) sleep pattern, while 24.8% were monophasic sleepers. Additionally, 8.5% followed a biphasic (post-down) pattern, and 7.2% adhered to a polyphasic sleep pattern.
-web-resources/image/OS-OMJ-D-25-00262_Table-2.png)
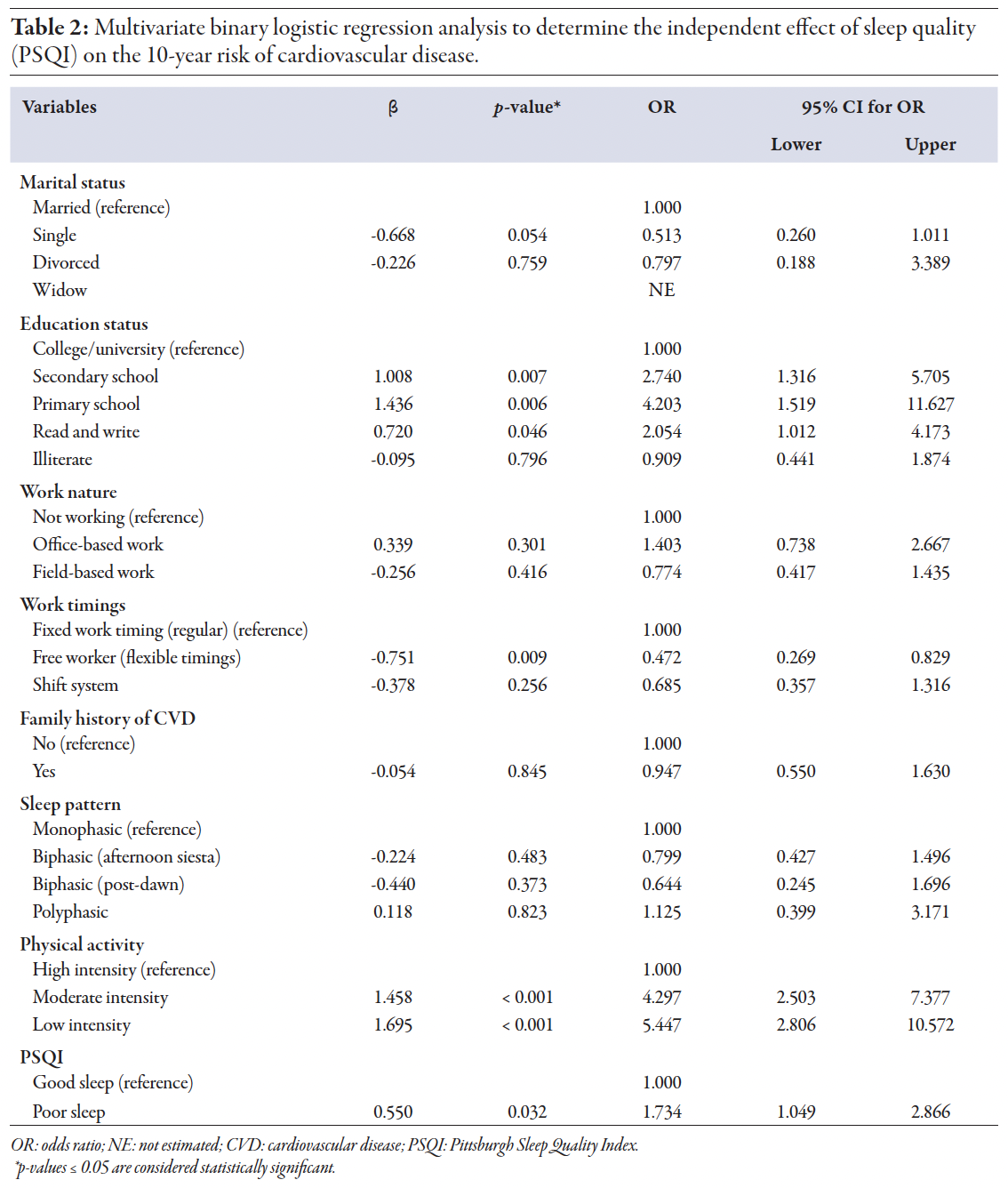 Table 2
Table 2
FRS showed that 66.5% of participants had a moderate to high 10-year CVD risk (≥ 10%). The analysis showed a significant association between sleep quality and an increased 10-year risk of CVD [Figure 1]. Participants with poor sleep quality had a 73.4% higher risk of developing CVD over 10 years compared to those with good sleep quality. This finding was supported by both the chi-square test results and multivariate logistic regression analysis (odds ratio (OR) = 1.734, 95% CI: 1.049–2.866; p = 0.032) [Table 2].
-web-resources/image/OS-OMJ-D-25-00262_Figure-1.png)
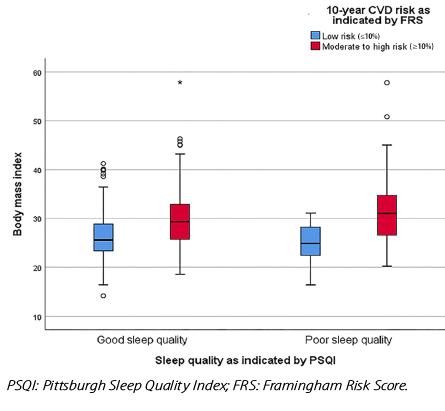 Figure 1: Impact of body mass index on sleep quality and 10-year cardiovascular disease (CVD) risk.
Figure 1: Impact of body mass index on sleep quality and 10-year cardiovascular disease (CVD) risk.
The 10-year CVD risk was significantly associated with education, work timings, and physical activity. However, the distribution of CVD risk among different sleep patterns: biphasic (afternoon siesta), biphasic (post-dawn), and polyphasic, showed no significant association with 10-year CVD risk (p = 0.483, 0.373, and 0.823, respectively), indicating that sleep patterns do not significantly impact the 10-year risk of developing CVD in this study
population [Table 2].
We observed a significant association between work timings and the 10-year risk of developing CVD. Individuals with flexible work timings had a lower CVD risk, with chi-squared analysis confirming that these differences were statistically significant (p = 0.009). Logistic regression further supported that flexible work timings were associated with a reduced risk of CVD (OR = 0.514;
p = 0.010) [Table 3].
-web-resources/image/OS-OMJ-D-25-00262_Table-3.png)
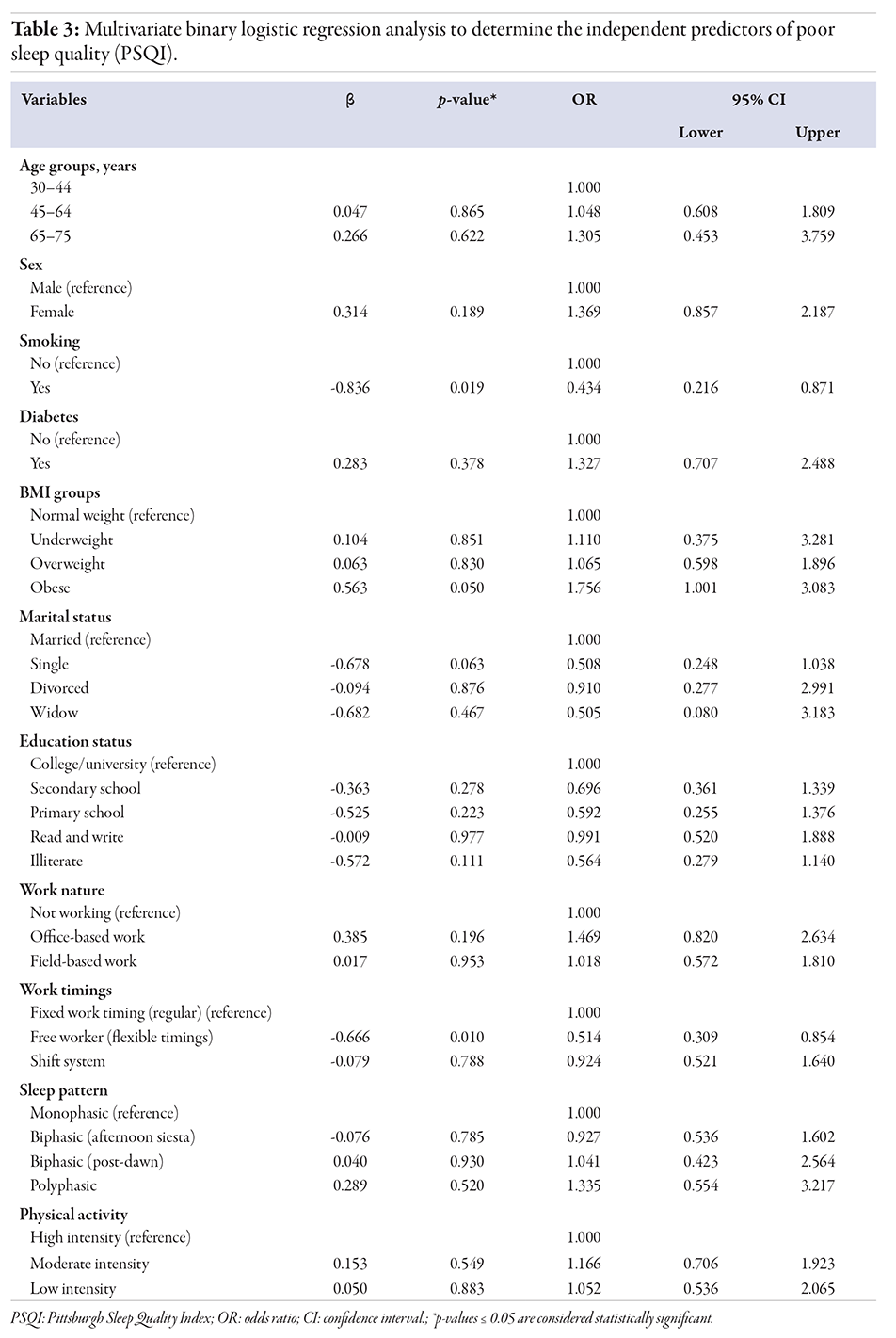 Table 3
Table 3
BMI and work timings emerged as significant independent predictors of sleep quality. Obese participants had a 75.6% higher risk of poor sleep quality compared to those with normal weight (OR = 1.756, 95% CI: 1.001–3.083; p = 0.050) [Figure 2].
-web-resources/image/OS-OMJ-D-25-00262_Figure-2.jpg)
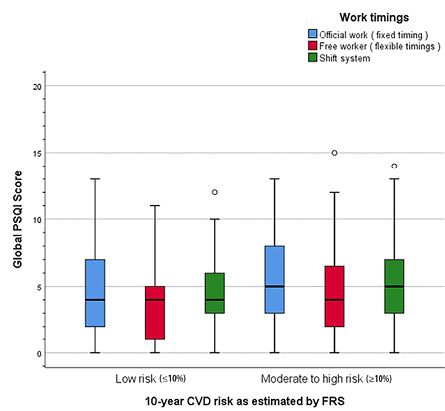 Figure 2: Impact of work timings on sleep quality and 10-year cardiovascular disease (CVD) risk.
Figure 2: Impact of work timings on sleep quality and 10-year cardiovascular disease (CVD) risk.
Discussion
We sought to examine the relationship between sleep quality and the 10-year risk of developing CVD among Omani adults attending primary healthcare centers in Muscat, Oman, with an emphasis on whether poor sleep quality serves as a significant predictor of elevated CVD risk. Additionally, the study aimed to assess sleep quality, sleep patterns, and the 10-year CVD risk, as well as explore the association between different sleep patterns (monophasic, biphasic, and polyphasic) and CVD risk. The study further examined the impact of work timing and work nature on cardiovascular health and its relationship with sleep quality. Sociodemographic factors, such as education level, were also assessed for their influence on sleep quality and subsequent CVD risk, along with exploring the relationship between BMI and sleep quality as a potential modifier of CVD risk.
The average nocturnal sleep duration in our study was 6.8 hrs, aligning with findings from recent regional studies, which reported approximately 7 hrs of sleep among Omani adults and 7.2 hrs among individuals in Saudi Arabia who adhere to the dawn (Fajr) prayer.3,13 A study in Kuwait further supported this pattern, reporting a mean sleep duration of 6.28 hrs using the PSQI and 6.7 hrs with actigraphy.14 These results suggest that the sleep durations in our sample are consistent with regional patterns influenced by cultural and religious practices, such as the Fajr prayer, which can disrupt consolidated sleep.3,13,14 The Kuwait study highlights that differences in sleep duration between our study and earlier research in Oman may be due to the more accurate measurements provided by actigraphy compared to the self-reported methods used in our study.14
Our findings show that most participants followed a biphasic sleep pattern, aligning with the traditional Mediterranean siesta and common Middle Eastern sleep habits.3,13 We also observed a biphasic post-dawn sleep pattern, with sleep episodes before and after the Fajr prayer. In contrast, a study conducted in Brazil, a region with different cultural, religious, and lifestyle contexts, found that monophasic sleep was predominant, with little evidence of biphasic or polyphasic sleep.12 This contrast highlights how cultural, religious, and lifestyle factors uniquely shape sleep behaviors in different regions.
Our study demonstrates a significant association between sleep quality and an increased 10-year risk of CVD, aligning with findings from a similar study conducted among female nurses in Hong Kong.15 In our research, participants with poor sleep quality had a 73.4% higher risk of developing CVD over 10 years compared to those with good sleep quality (OR = 1.734, 95% CI: 1.049–2.866; p = 0.032). The Hong Kong study also found that female nurses with sleep disturbances had a higher CVD risk (OR = 1.82, 95% CI: 1.04–3.18), similar to our observed OR.15 Numerous other studies have supported this association, showing that poor sleep quality, insomnia, and short sleep duration are consistent predictors of increased cardiovascular risk. Other studies reinforce the evidence that poor sleep is a significant contributor to elevated CVD risk across different populations.1,6,16 Several mechanisms may explain this relationship, as poor sleep quality is known to contribute to various physiological disruptions, including increased sympathetic nervous system activity, elevated blood pressure, inflammation, and metabolic dysregulation—all of which are well-established risk factors for CVD.11,17
Additionally, poor sleep can exacerbate conditions like obesity and T2DM, further increasing the likelihood of cardiovascular issues.11 A meta-analysis exploring the impact of sleep deprivation on CVD risk found that sleep deprivation significantly increases the risk of CVD by contributing to inflammation, hypertension, and insulin resistance, all of which are critical contributors to the development and progression of atherosclerosis.18
While our study found a general association between poor sleep quality and increased CVD risk, the Hong Kong study also highlighted specific components of sleep quality, such as daytime dysfunction, which had a more complex relationship with CVD risk.15 Interestingly, higher daytime dysfunction scores in the Hong Kong study were associated with a lower CVD risk—contrary to the expected relationship between poor sleep quality and cardiovascular health.15 This finding suggests that while overall sleep quality is an essential factor, individual components of sleep may interact with cardiovascular risk factors in nuanced ways.15 This subtle interaction calls for more targeted research to unravel these relationships, particularly how different aspects of sleep can independently or synergistically influence cardiovascular outcomes.
Our results indicated that the distribution of CVD risk among different sleep patterns did not show a statistically significant difference: biphasic (afternoon siesta), biphasic (post-dawn), and polyphasic (with p-values of 0.483, 0.373, 0.823, respectively) [Table 2], suggesting that sleep patterns alone do not significantly influence the 10-year risk of developing CVD. In the Omani study by Al-Abri et al, different sleep patterns were identified, including monophasic, biphasic-siesta, biphasic-dawn, and polyphasic patterns.3 Although these patterns varied significantly in terms of sleep duration and timing, the study did not find a clear association between these sleep patterns and health outcomes related to sleep.3 The lack of a significant difference in CVD risk across sleep patterns in our study may be attributable to the complex interplay of factors influencing cardiovascular health. The Omani study highlighted that lifestyle, environmental factors, and cultural practices, such as afternoon siestas, might mitigate the potential adverse effects of segmented or polyphasic sleep patterns.3
Our statistical analysis revealed no significant association between sleep patterns and sleep quality (p = 0.529) [Table 4]. We also did not identify any significant predictors of poor sleep quality across different sleep patterns, although there was a slight, non-significant trend toward poorer sleep quality among participants with a polyphasic sleep pattern (OR = 1.335; p = 0.520). These findings suggest that, in our sample, sleep patterns do not significantly impact sleep quality as measured by the PSQI, aligning with recent studies that have shown no strong link between sleep patterns and sleep quality.3,13 Notably, no previous studies have specifically examined the relationship between monophasic, biphasic (afternoon siesta and post-dawn), and polyphasic sleep patterns and sleep quality.
-web-resources/image/OS-OMJ-D-25-00262_Table-4.png)
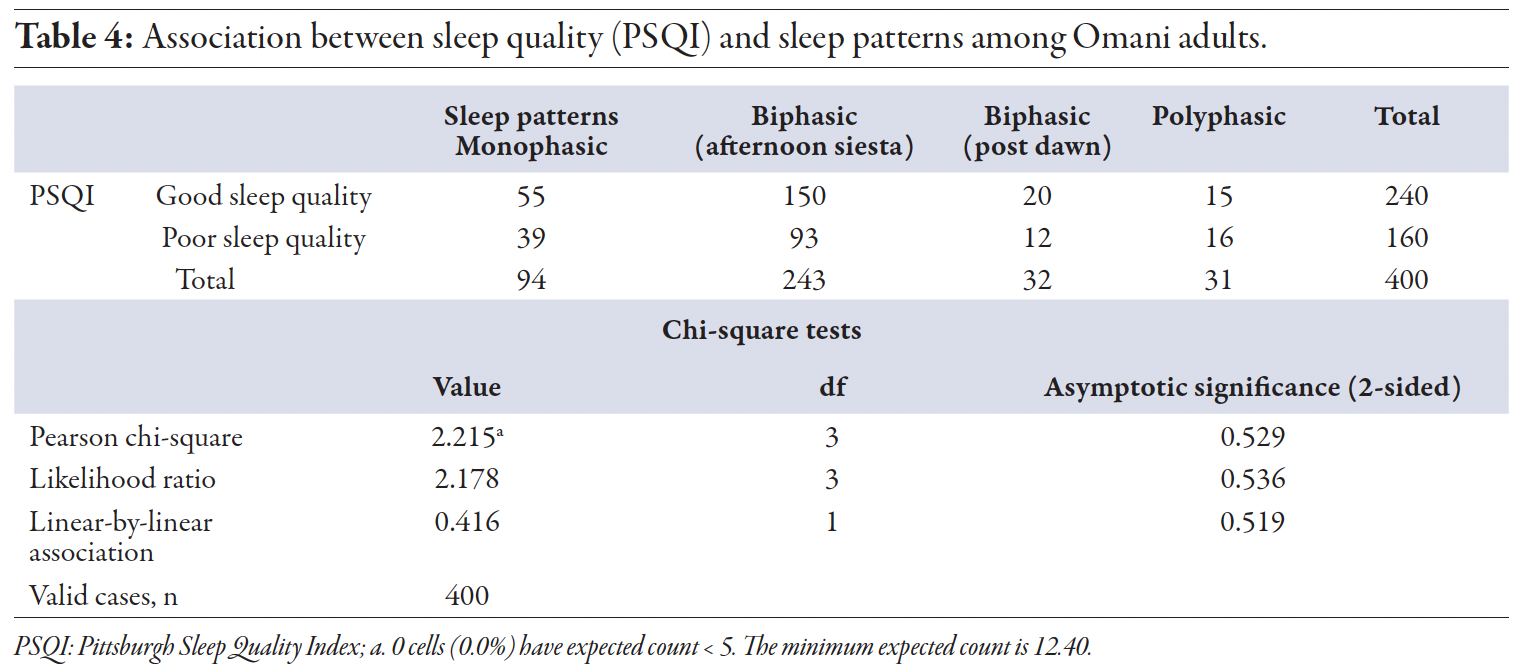 Table 4
Table 4
Our study revealed a significant association between work timings and the 10-year risk of developing CVD (p = 0.009). Individuals with flexible work timings showed a significantly lower CVD risk (OR = 0.472; p = 0.009) [Table 2]. Additionally, a significant association between work timings and sleep quality was observed (p = 0.010) [Table 3]. Further analysis showed that individuals with flexible work timings had significantly lower odds of experiencing poor sleep quality (OR = 0.514, 95% CI: 0.309–0.854; p = 0.010), indicating that flexibility in work hours may improve sleep quality.
These findings align with existing research exploring the impact of work schedules on individual health, demonstrating that flexible work timings are associated with improved health outcomes, particularly in reducing cardiovascular risk.19–22 Improved sleep quality, circadian rhythm stability, and reduced stress levels are likely mechanisms through which flexible work timings exert their protective effects on cardiovascular health. In Japan, individuals with flexible schedules reported better sleep quality compared to those with fixed hours.22 Joyce et al,19 highlighted that flexible working conditions improve sleep quality and reduce psychological stress, both of which are linked to lower cardiovascular risk.Similarly, Haley and Miller showed that flexible schedules lower stress and improve sleep quality, indirectly contributing to better cardiovascular health.21 Kelly et al,20 provided evidence that flexible work arrangements improve sleep and reduce stress, enhancing cardiovascular outcomes by promoting better alignment with circadian rhythms.
Our study also showed that individuals with lower education levels are at a significantly higher risk of developing CVD over a 10-year period. Educational attainment is a well-established determinant of CVD risk.20 Studies suggest that individuals with higher education levels tend to have better cardiovascular health, partly due to greater health literacy, healthier lifestyle choices, and better access to healthcare resources.23,24 People with higher educational levels are more likely to have stable jobs, regular work hours, and better living conditions, which contribute to better sleep.23 Conversely, those with lower education levels may experience job insecurity, irregular work hours, and higher stress levels, leading to poorer sleep quality.23 This suggests that lower educational levels not only directly contribute to higher CVD risk through lifestyle and healthcare access but also indirectly through thier impact on sleep quality.
Our findings strongly suggest that increasing the intensity of physical activity is associated with a lower risk of developing CVD over 10 years. This aligns with extensive research showing that regular, vigorous physical activity plays a crucial role in reducing cardiovascular risk factors, including hypertension, obesity, and poor lipid profiles.25 Encouraging higher levels of physical activity could be an effective strategy in CVD prevention.
Most research has focused on the effects of sleep duration on physiological changes like obesity and metabolic markers. Still, there is limited investigation into the relationship between overall sleep quality and other anthropometric measures in the absence of sleep apnea, particularly among the general adult population of Oman.4,9,14,26 One of the objectives of our study was to assess the independent effects of sociodemographic factors and health-related issues like obesity on sleep quality in the adult Omani population. In our study, 60.0% of participants reported good sleep quality, compared to 55% in a previous Omani study.3 The slight variation may be due to broader work types and flexible schedules in our sample, which positively influenced sleep quality. The previous Omani study did not examine work schedules in detail, which may have contributed to their lower percentage of good sleepers. Additionally, our study used subjective self-reports, whereas the Omani study employed actigraphy, which may have detected sleep disruptions not captured in self-assessments, further explaining the differences in findings.
The higher prevalence of poor sleep quality observed in our study and other regional studies, such as the Kuwaiti study, may be attributed to the greater prevalence of obesity in Gulf countries.14 In our study, 40.0% of participants reported poor sleep quality, with BMI emerging as a significant independent predictor. Obese participants faced a 75.6% higher risk of poor sleep quality compared to those with normal weight (OR = 1.756, 95% CI: 1.001–3.083; p = 0.050). Similarly, the Kuwaiti study reported a higher prevalence of poor sleep quality (59.6%) and a positive correlation between BMI and sleep quality (r = 0.348, p ≤ 0.0001).14 The rising trend toward higher BMI in Middle Eastern countries has been linked to an increasing prevalence of obesity, driven by the adoption of Westernized diets high in fat, sugar, and salt, increased eating outside the home, and low physical activity levels. These factors were also believed to influence both obesity and sleep quality.14,27 Other studies across different populations and settings have consistently demonstrated a strong relationship between sleep quality and obesity.28–30
The relationship between poor sleep quality and obesity appears to be bidirectional, although the underlying mechanisms remain unclear.31 Even without sleep apnea, individuals with obesity may experience impaired sleep quality and duration.32 Poor sleep quality can increase the risk of obesity through its impact on metabolism, hormone secretion, and appetite regulation.28,29 Disruptions in the secretion of hormones such as ghrelin and leptin during sleep restriction can lead to an energy imbalance, affecting food choices and calorie intake.28,29 Additionally, poor sleep quality contributes to fatigue, reducing physical activity, and promoting abdominal fat accumulation. Overall, these factors may lead to an imbalance between energy intake and expenditure, thereby promoting weight gain.28,29
Obesity, in turn, significantly impacts sleep quality, mainly through the accumulation of excessive adipose tissue, which narrows and obstructs the upper airway, contributing to sleep dysfunction, a key component of sleep quality assessment.30 While this is more common in cases of severe obesity, sleep dysfunction also affects individuals with grade I obesity or overweight, suggesting additional contributing mechanisms beyond airway obstruction.30 Visceral adipose tissue plays a significant role in releasing inflammatory cytokines such as IL-1, IL-6, and TNF-α, which influence sleep regulation, particularly slow-wave sleep. A previous study found that waist circumference, an indirect measure of visceral fat, was higher in individuals with sleep dysfunction and positively correlated with sleep quality as measured by the PSQI.30 In contrast, only a few studies found no significant relationship between sleep quality and obesity, indicating that this association may vary depending on other individual or contextual factors.29,33
Our study did not find a significant association between sleep quality and work nature (office-based or field-based; p = 0.499) [Table 5]. This is consistent with findings from Kuwait, which showed no differences in sleep quality between desk and non-desk jobs.14 These findings suggest that other factors, such as work schedule flexibility and individual health behaviors, may be more influential in determining sleep quality than the physical nature of one’s work.
-web-resources/image/OS-OMJ-D-25-00262_Table-5.png)
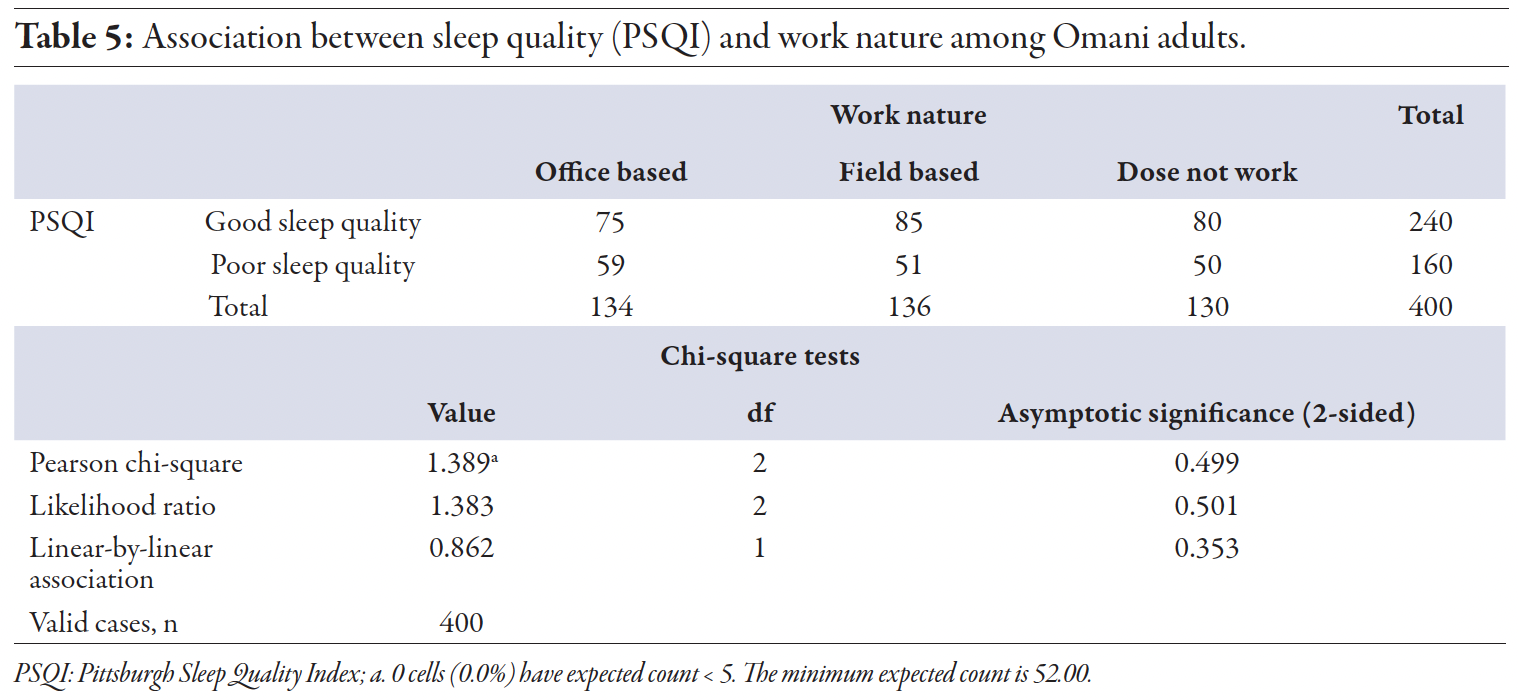 Table 5
Table 5
This study contributes to the growing body of evidence on the relationship between sleep quality, CVD risk, work schedules, and obesity within the Middle Eastern population, with a specific focus on the Omani context. However, there are several limitations in our study. First, the cross-sectional design of this study limits the ability to infer causality between variables. The associations observed, such as those between flexible work timings and improved sleep quality or between obesity and poor sleep quality, may reflect bidirectional influences that cannot be clarified without longitudinal data. Additionally, the use of self-reported data for sleep quality through the PSQI introduces the potential for recall bias, which may affect the accuracy of the findings. The convenience sampling of participants from three healthcare centers in Muscat may limit the generalizability of the results to the broader population. While cultural and religious practices unique to the region influence sleep patterns, their wider impact on CVD outcomes remains understudied. Finally, the use of the non-laboratory FRS for CVD risk estimation, while valid, lacks detailed clinical markers, and the exclusion of participants with established CVD or obstructive sleep apnea may have resulted in an underestimation of the associations observed.
Conclusion
This study highlights the critical role of sleep quality in predicting the 10-year risk of CVD among Omani adults. Poor sleep quality was found to significantly elevate CVD risk, whereas flexible work timings demonstrated a protective effect. The lack of a significant association between sleep patterns and CVD risk suggests that sleep quality, rather than specific sleep patterns, plays a more influential role in cardiovascular health. Furthermore, lower educational attainment was identified as a significant contributor to increased CVD risk, and obesity was linked to both poor sleep quality and elevated CVD risk. These findings underscore the importance of public health initiatives aimed at promoting better sleep hygiene, particularly targeting vulnerable groups, including individuals with shift work schedules, lower educational backgrounds, and higher BMI. Addressing these factors may be key to mitigating long-term cardiovascular risks in
the population.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study. The data for this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
references
- 1. Covassin N, Singh P. Sleep duration and cardiovascular disease risk: epidemiologic and experimental evidence. Sleep Med Clin 2016 Mar;11(1):81-89.
- 2. National Sleep Foundation. How much sleep do you really need? [cited 2024 Oct]. Available from: https://www.thensf.org/how-many-hours-of-sleep-do-you-really-need/.
- 3. Al-Abri MA, Al Lawati I, Zadjali F, Ganguly S. Sleep patterns and quality in Omani adults. Nat Sci Sleep 2020 Apr;12:231-237.
- 4. Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011 Jun;32(12):1484-1492.
- 5. Yan B, Yang J, Zhao B, Fan Y, Wang W, Ma X. Objective sleep efficiency predicts cardiovascular disease in a community population: the sleep heart health study. J Am Heart Assoc 2021 Apr;10(7):e016201.
- 6. Wang Z, Yang W, Li X, Qi X, Pan KY, Xu W. Association of sleep duration, napping, and sleep patterns with risk of cardiovascular diseases: a nationwide twin study. J Am Heart Assoc 2022 Aug;11(15):e025969.
- 7. Addo PN, Mundagowa PT, Zhao L, Kanyangarara M, Brown MJ, Liu J. Associations between sleep duration, sleep disturbance and cardiovascular disease biomarkers among adults in the United States. BMC Public Health 2024 Apr;24(1):947.
- 8. Full KM, Malhotra A, Gallo LC, Kerr J, Arredondo EM, Natarajan L, et al. Accelerometer-measured sleep duration and clinical cardiovascular risk factor scores in older women. J Gerontol A Biol Sci Med Sci 2020 Sep;75(9):1771-1778.
- 9. Al-Abri MA, Al Lawati I, Al Zadjali F. Association of elevated glycated hemoglobin and obesity with afternoon napping for more than 1 h in young and middle-aged healthy adults. Front Psychiatry 2022 Oct;13:869464.
- 10. Sleep Foundation. Sleep dictionary. [cited 2024 Oct]. Available from: https://www.sleepfoundation.org/how-sleep-works/sleep-dictionary.
- 11. Al-Abri MA, Jaju D, Al-Sinani S, Al-Mamari A, Albarwani S, Al-Resadi K, et al. Habitual sleep deprivation is associated with type 2 diabetes: a case-control study. Oman Med J 2016 Nov;31(6):399-403.
- 12. Matuzaki L, Santos-Silva R, Marqueze EC, de Castro Moreno CR, Tufik S, Bittencourt L. Temporal sleep patterns in adults using actigraph. Sleep Sci 2014 Sep;7(3):152-157.
- 13. Bahammam AS, Sharif MM, Spence DW, Pandi-Perumal SR. Sleep architecture of consolidated and split sleep due to the dawn (Fajr) prayer among Muslims and its impact on daytime sleepiness. Ann Thorac Med 2012 Jan;7(1):36-41.
- 14. Al-Rashed F, Sindhu S, Al Madhoun A, Alghaith A, Azim R, Al-Mulla F, et al. Short sleep duration and its association with obesity and other metabolic risk factors in Kuwaiti urban adults. Nat Sci Sleep 2021 Jul;13:1225-1241.
- 15. Yan LJ, Xie YJ. Associations between sleep quality and 10-year cardiovascular disease risk among female nurses in Hong Kong: a cross-sectional study. J Cardiovasc Nurs 2022 May-Jun;37(3):E22-E31.
- 16. Bertisch SM, Pollock BD, Mittleman MA, Buysse DJ, Bazzano LA, Gottlieb DJ, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: sleep heart health study. Sleep 2018 Jun;41(6):zsy047.
- 17. Qasrawi SO. BaHammam AS. Role of sleep and sleep disorders in cardiometabolic risk: a review and update. Curr Sleep Med Rep 2024;10(1):34-50.
- 18. Belloir J, Makarem N, Shechter A. Sleep and circadian disturbance in cardiovascular risk. Curr Cardiol Rep 2022 Dec;24(12):2097-2107.
- 19. Joyce K, Pabayo R, Critchley JA, Bambra C. Flexible working conditions and their effects on employee health and wellbeing. Cochrane Database Syst Rev 2010 Feb;2010(2):CD008009.
- 20. Kelly EL, Moen P, Tranby E. Changing workplaces to reduce work-family conflict: schedule control in a white-collar organization. Am Sociol Rev 2011 Apr;76(2):265-290.
- 21. Haley MR, Miller LA. Correlates of flexible working arrangements, stress, and sleep difficulties in the US workforce: does the flexibility of the flexibility matter? Empir Econ 2015;48:1395-1418.
- 22. Yoshida A, Asakura K, Imamura H, Mori S, Sugimoto M, Michikawa T, et al. Relationship between working hours and sleep quality with consideration to effect modification by work style: a community-based cross-sectional study. Environ Health Prev Med 2024;29:19.
- 23. Berry-Cabán CS, Beltran TA, Han RH, McKiernan SP, Choi YS. Sweet dreams, bright futures: the relationship between sleep duration and health, income and education. Discov Soc Sci Health 2023;3(1):26.
- 24. Jaspan VN, Greenberg GS, Parihar S, Park CM, Somers VK, Shapiro MD, et al. The role of sleep in cardiovascular disease. Curr Atheroscler Rep 2024 Jul;26(7):249-262.
- 25. Valenzuela PL, Ruilope LM, Santos-Lozano A, Wilhelm M, Kränkel N, Fiuza-Luces C, et al. Exercise benefits in cardiovascular diseases: from mechanisms to clinical implementation. Eur Heart J 2023 Jun;44(21):1874-1889.
- 26. Krittanawong C, Tunhasiriwet A, Wang Z, Zhang H, Farrell AM, Chirapongsathorn S, et al. Association between short and long sleep durations and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care 2019 Dec;8(8):762-770.
- 27. Kilpi F, Webber L, Musaigner A, Aitsi-Selmi A, Marsh T, Rtveladze K, et al. Alarming predictions for obesity and non-communicable diseases in the Middle East. Public Health Nutr 2014 May;17(5):1078-1086.
- 28. Li B, Liu N, Guo D, Li B, Liang Y, Huang L, et al. Association between sleep quality and central obesity among southern Chinese reproductive-aged women. BMC Womens Health 2021 Aug;21(1):280.
- 29. Rahe C, Czira ME, Teismann H, Berger K. Associations between poor sleep quality and different measures of obesity. Sleep Med 2015 Oct;16(10):1225-1228.
- 30. Muscogiuri G, Barrea L, Aprano S, Framondi L, Di Matteo R, Laudisio D, et al; on behalf of the OPERA PREVENTION Project. Sleep quality in obesity: does adherence to the mediterranean diet matter? Nutrients 2020 May;12(5):1364.
- 31. Muscogiuri G, Barrea L, Annunziata G, Di Somma C, Laudisio D, Colao A, et al. Obesity and sleep disturbance: the chicken or the egg? Crit Rev Food Sci Nutr 2019;59(13):2158-2165.
- 32. Resta O, Foschino Barbaro MP, Bonfitto P, Giliberti T, Depalo A, Pannacciulli N, et al. Low sleep quality and daytime sleepiness in obese patients without obstructive sleep apnoea syndrome. J Intern Med 2003 May;253(5):536-543.
- 33. He J, Fan Y, Zhang L, Li C, Guo F, Zhu J, et al. Habitual night sleep duration is associated with general obesity and visceral obesity among Chinese women, independent of sleep quality. Front Public Health 2023 Jan;11:1053421.