Hyperinfection by Strongyloides Stercoralis
Uday A. Gokhale, G. Rajasekharan Pillai, Said Al-Mammari, Dhia Al-Layla
Gokhale UA, et al. OMJ. 25, (2010); doi:10.5001/omj.2010.47
ABSTRACT
Strongyloides stercoralis is an intestinal nematode with a complex life cycle capable of a free living cycle, a parasitic cycle and auto-infection. It is usually asymptomatic in a healthy host but causes life threatening hyperinfection involving multiple organs in immunocompromised patients. However, reports of gastric involvement are very rare. This is a report of gastric strongyloidiasis with evidence of hyperinfection in an elderly Omani patient on chronic corticosteroid therapy for chronic obstructive pulmonary disease (COPD) and H-2 blocker for dyspepsia.
From the Department of Pathology , Sultan Qaboos Hospital, Salalah, Sultanate of Oman.
Received: 05 Feb 2010
Accepted: 11 Mar 2010
Address of Corresponding Author: Dr. Uday Arun Gokhale, Department of Pathology, Sultan Qaboos Hospital, Sultanate of Oman.
E-mail: uday_gokhale@rediffmail.com
Gokhale UA, et al. OMJ. 25, (2010); doi:10.5001/omj.2010.47
INTRODUCTION
Strongyloides stercoralis is an intestinal nematode of humans. It is prevalent throughout the tropical, subtropical and temperate regions.1 Man is infected by filariform larvae of Strogyloides stercoralis. After penetrating the skin, they enter cutaneous vessels and are carried to the lungs. The larvae then migrate via the respiratory tree, and are swallowed with sputum and finally reach and mature into adult egg laying females in the mucosa of the small intestine, especially the duodenum and upper jejunum. The eggs mature rapidly into rhabditiform larvae and are excreted in the stool specimen.2 The host-parasite relationship can be altered due to certain predisposing factors such as corticosteroid therapy, anticancer drugs, malnutrition among other factors, giving rise to a pathological state of hyperinfection causing manifestations in many organs.3 However, the stomach involvement is very rare.4
A 74 year old Omani male, living in Dalqut, a small town of Dhofar province of the Sultanate, was admitted to the Gastroenterology unit of Sultan Qaboos Hospital in May 2009, with complaints of nausea, vomiting, poor intake and abdominal pain. The patient looked ill and emaciated. His vital signs were stable. The physical examination was normal except for epigastric tenderness.
The patient was a known case of COPD (Chronic obstructive pulmonary disease) for many years and was receiving corticosteroid therapy. He had complaints of dyspepsia since 2000 and was receiving intermittent treatment with H-2 blockers (ranitidine) and occasionally by proton pump inhibitor, (omiprazole). Recently, in January 2009 he was admitted with pyogenic meningitis caused by gram-negative bacteria. He was cured of illness but his general condition continued to deteriorate and he was re-hospitalized in April 2009 with colicky abdominal pain, vomiting, diarrhea and exacerbation of COPD. He was treated with antibiotics and received oral prednisolone and ranitidine. Stool examination performed at that time did not show any parasite.
Laboratory investigations during the current admission revealed 12.2 gms/dl of hemoglobin and leukocyte count was 15,900/Ál with 75% of neutrophils. There was no eosinophilia (2.58%). Biochemical investigations showed hypoproteinemia (55.4 g/L, Normal = 66 to 87 g/L), hypoalbuminemia (15.8 g/l, Normal = 35 to 50 g/L) and hyponatremia (123 mmol/L, Normal = 135 to 145 mmol/L). Sputum culture showed growth of E. coli while the urine culture was negative. The patient was negative for HBsAg, anti HCV and HIV. A chest radiograph showed features of COPD in both lungs. Ultrasound examination of the abdomen showed thickened wall of the small bowel.

On upper gastrointestinal endoscopy, the gastric mucosa showed erosions, erythema and whitish mottled surface with an elevated lesion on the greater curvature at the junction of the antrum and pylorus, (Fig. 1). The duodenum also showed edema, erosions and erythema. Fiberoptic biopsy from the raised lesion in the stomach was obtained, and the formalin fixed specimen showed three fragments of light brown colored tissue each measuring 2 mm in diameter. No biopsy from the duodenum was taken.
The histological examination revealed superficial fragments of gastric antral mucosa showing surface erosions, congestion, many ectatic blood vessels and dense infiltration by lymphocytes, plasma cells, neutrophils and few eosinophils in the lamina propria. Furthermore, the gastric mucosa showed numerous cross-sections of adult worms, eggs and rhabditiform larvae of Strongyloides stercoralis in the glands and crypts (Figs. 2, 3, 4). Few adult worms were seen on the surface. However, no H. pylori demonstrated.
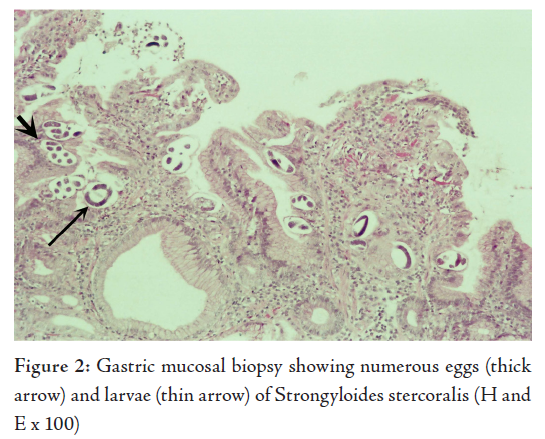
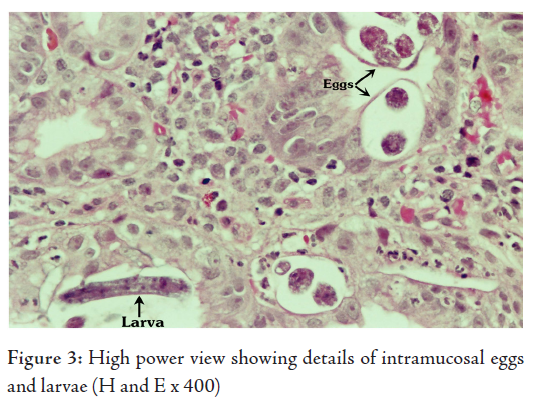
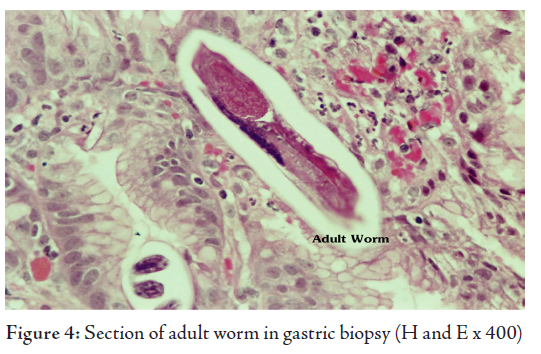
The diagnosis of hyperinfection by Strongyloides stercoralis was made in this elderly patient after demonstrating the parasite in his gastric biopsy, E.coli in his sputum sample and correlating with the past history of gram negative meningitis and gastrointestinal symptoms treated with ranitidine and omiprazole in a background of longterm corticosteroid therapy for COPD.
The patient decided to get further treatment from a centre outside the country. A repeat biopsy was taken there and the diagnosis was confirmed. The patient received ivermectin and he is symptomatically better since then.
DISCUSSIONStrongyloidiasis is a worldwide parasitic infection affecting approximately 75 million people most densely distributed in areas characterized by high temperature, humidity and poor hygiene.5 The most endemic areas are South-East Asia, Latin America, Sub-Saharan Africa and pockets in South-Eastern United States.6 In Oman, it has a very low prevalence with sporadic cases reported in the Dhofar region of the Sultanate. 7
Low socio-economic status, alcoholism, being Caucasean, male gender and occupations requiring contact with soil contaminated by human waste such as farming, coal mining etc. increase the risk of infection.1 The infection occurs after skin penetration by filariform larvae from the soil or by larvae on fomites, food, waste or feces. Human to human spread has been reported after anal sexual contact. 4
Strongyloides stercoralis is unique among common helminthes for its potential for autoinfection and persistence. The rhabditiform larvae formed are transformed into infectious filariform larvae before being excreted in stools. They re-infect the host through the intestinal mucosa or perianal skin .8
Extraintestinal infections occur in the lungs, liver, spleen, pancreas, gall bladder, kidneys, thyroid, brain and meninges, skin, mesenteric lymph nodes, ovaries and skeletal muscle in hyperinfection.2,8 Gastric involvement has rarely been reported. While the stomach is not a congenial site for Strogyloides stercoralis, reduced gastric secretion may favor infection and invasion of the stomach. It has been suggested that the organisms reach the stomach of the patient through sputum or via retrograde migration from the proximal small intestine.4
Hyperinfection occurs in the context of immunosuppression due to disease or therapy, particularly with corticosteroids.9 Other risk factors are old age, underlying chronic lung disease, malignancy, acquired immunodeficiency syndrome, achlorhydria, use of H-2 blockers, gastrointestinal disorders and malnutrition.2,4 It has been reported that immunosuppressive therapy increases the possibility of an infection and that achlorhydria (often brought about by treatment with histamine-2 blockers or proton pump inhibitors) may facilitate gastric strongyloidiasis.2 The age, chronic corticosteroid therapy for COPD and intermittent use of H-2 blocker for dyspepsia probably were the risk factors in the studied patient.
Secondary bacterial infection with bacteremia and meningitis may occur due to breach of the bowel mucosa by the worms allowing gut organisms access to the blood stream.8,10 This patient had pyogenic meningitis two months earlier, probably due to the same reason.
The laboratory diagnosis of strongyloidiasis is usually made by demonstrating rhabditiform larvae in fecal specimen. The sensitivity of a single stool sample microscopy is only 30% and a routine stool examination may fail to find larvae when the intestinal worm burden and output of larvae is minimum. Larvae may not be detected in a cursory examination of small quantity of feces.2,4 To improve the chances of finding the parasite, repeated examination of stool specimen should be performed, preferably with concentration techniques.11 No larvae were found in the stool sample examined during the previous admission for gastroenteritis a month prior to the endoscopic biopsy.
Culture techniques such as Baermann method, Harada-Mori filter paper culture and the nutrient agar plate method are reported to be much more sensitive than routine stool microscopy, with an accuracy rate up to 96% for the agar plate method. However, these methods are expensive and time consuming.8
The ELISA (Enzyme linked immunosorbent assay) test is somewhat useful in detecting antibodies (IgG) to filariform larvae. But its specificity is compromised when cross reactive antibodies are present from other helminth infections. Moreover, it is often difficult to differentiate between past and current infections.12
In general, the endoscopic findings of strongyloidiasis are not very specific. The stomach may show thickened folds and mucosal erosions. In the duodenum, the usual findings are edema, brown discoloration of the mucosa, erythematous spots, subepithelial hemorrhages and megaduodenum.8 The histological diagnosis from the biopsies or surgical specimens is usually incidental in patients undergoing gastrointestinal endoscopy for other clinically suspected conditions.5 In the studied case too, the biopsy was taken as the patient had a history of epigastric pain and dyspepsia. Different stages of maturation of the parasite can be demonstrated on microscopy of the biopsy material. Adult female worms, eggs and larvae are seen in the epithelium of the crypts with acute or chronic inflammation in the mucosa with eosinophils.2,8 Damage of the surface epithelium with hyperplastic reactive changes are noted in most of the cases.4,5
Eosinophilia is common in strongyloidiasis ranging from 25 -35% in acute cases and 6-8% in chronic cases. But sometimes, eosinophil count may be low in some immunosuppressed conditions such as corticosteroid administration as was the case in this patient. Absence of eosinophilia indicates poor prognosis. 2
Although thiabendazole was the treatment of choice for strongyloidosis and still a first choice of drug, it has now been replaced by ivermectin.8 It is fairly well tolerated and has a higher cure rate than thiabendazole.12 Other drugs such as mebendazole and albendazole have variable therapeutic efficacy.4
CONCLUSIONStrongyloidiasis is a curable disease. Early diagnosis and appropriate therapy will reduce the mortality and morbidity.3 Unless severely infected, the clinical signs and symptoms are generally not severe and frequently nonspecific. The infection can be overlooked by the patient and physician.4 Furthermore, it may turn fatal if the patient becomes immunocompromised. Hence, it is prudent to look for strongyloidiasis in high risk patients if immunosuppression (e.g. long term corticosteroids treatment) is anticipated.
We are grateful to Dr. M.S. Zulfiquar Ahmed for helping us with the computer graphics.
-
Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev 2004; 17:208-217.
-
Kim J, Joo HS, Kim DH, Lim H, Kang YH, Kim MS. A case of gastric strongyloidiasis in a Korean patient. Korean J Parasitol. 2003; 41:63-67.
-
Nam SC, Han MH, Kim YS, Kum YS, Suh IS, Bae HI. Two cases of Strongyloidiasis diagnosed by colonoscopic biopsy. Korean J Parasitol. 2007; 41:343-346.
-
Yaldiz M, Hakverdi S, Aslan A, Temiz M, Culha G. Gastric infection by Strongyloides stercoralis: A case report. Turk J Gastroenterol. 2009; 20:48-51.
-
Rivasi F, Pampiglione S, Boldorini R, Cardinale L. Histopathology of gastric and duodenal Strongyloides stercoralis locations in fifteen immunocompromised subjects. Arch Pathol Lab Med. 2006;130:1792-1798.
-
Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001; 33:1040-1047.
-
Scrimgeour EM, Mehta FR, Ali-Jaffer MS. Infections and tropical diseases in Oman: A review. Am J Trop Med Hyg. 1999; 61:920-925.
-
Maguire WF, Mintzer DM, Stopyra GA, Stern J. Strongyloidiasis diagnosed by endoscopic biopsy in a patient with multiple myeloma. Commun Oncol. 2006; 3:144-146.
-
Mani RK, Sardana R, Chawla R, Bansal A, Kanwar MS, Kansal S. Respiratory failure, coma and cutaneous lesions due to disseminated strongyloidiasis. Indian J Crit Med 2003; 7:132-136.
-
Bangs MJ, Sirait S, Purnomo, Maguire JD. Strongyloidiasis with gastric mucosal invasion presenting with acute interstitial nephritis. Southeast Asian J Trop Med Public Health. 2006; 37:641-647.
-
Bader Abdul KM, Cherian S, Hamdy Ibrahim AR. Hyperinfection with strongyloides stercoralis. Kuwait Medical Journal. 2002; 34:221-223.
-
Greiner K, Bettencourt J, Semolic C. Strongyloidiasis: A review and update by case example. Clin Lab Sci.2008; 21:82-88.