Sinonasal tumors are tumors that occur in the nasal cavity or paranasal sinuses (PNS). These tumors are rare and make up about only 3% of tumors in the upper respiratory tract.1 They are twice as common in males than females and are seen in the fifth and sixth decades of life. Human papillomavirus (HPV) 6 and 11, allergens, air pollution, and industrial carcinogens predispose to the development of benign lesions. Tobacco, alcohol, and occupational exposure to heavy metal particles (such as nickel and chromium), particularly for workers in the leather, textile, furniture, and wood industries, predispose the development of malignancies in the sinonasal tract. Patients with sinonasal tumors present with vague complaints like nasal obstruction, nasal congestion and discharge, headache and/or swelling and facial pain. Diagnosis begins with a thorough clinical history and physical examination. Computed tomography/magnetic resonance imaging (CT/MRI) scans are done to stage the tumor locally and to check for the presence of metastasis. Biopsy of the tumor is necessary to make a final diagnosis. Almost all the benign tumors have a tendency to recur with locally destructive capability and a 5–15% likelihood to progress to malignancy. Surgery (external or endoscopic) is the mainstay of treatment with or without radiation therapy and chemotherapy.1-3 Sinonasal tumors carry a poor prognosis, despite the early diagnosis, radical surgical resection, and strict follow-up.
In order to understand this entity better, we reviewed 610 sinonasal biopies/specimens.
Methods
A retrospective study was undertaken in the Department of Pathology of the Himalayan Institute of Medical Sciences, India, over a period of 10 years (2004–14). Clinical data was retrieved from histopathology requisition forms/hospital records of patients who had presented with a nasal mass. A CT scan of the nose and PNS was done in all cases, whereas MRI was carried out where indicated. All the specimens (biopsies/surgical specimens) were fixed in 10% formalin, embedded in paraffin, sectioned at 5 µ and stained with hematoxylin and eosin (H&E). Special stains (Ziehl-Neelson, Periodic acid-Schiff, and silver methenamine) were carried out for microorganisms where required. Immunohistochemistry in a few of the malignant tumors was also performed. The antibody panel chosen for round cell or undifferentiated malignant tumors included pan-cytokeratin (Pan-CK), neuron-specific enolase (NSE), leukocyte common antigen (LCA), HMB-45, and vimentin (BioGenex, California, US).
Results
A total of 610 lesions of the nose and PNS were studied over 10 years. Of these 435 (72.0%) were benign, 128 (21.2%) were malignant, and 47 (7.7%) were inflammatory/non-neoplastic. Of all the benign tumors, 21% were seen in the third decade of life while most of the malignant tumors were seen in the fifth decade of life, whereas 36% of inflammatory and non-neoplastic sinonasal lesions occurred in the 21–30 year age group [Figure 1]. The benign tumors showed a clear-cut male preponderance (3:1) while malignant tumors and inflammatory conditions showed an almost equal sex ratio. Most patients complained of vague signs and symptoms; however, those harboring a benign tumor presented with unilateral nasal obstruction (n=308/435, 70.0%) followed by sinusitis and headache. Most patients with malignant tumors presented with blood-stained nasal discharge (76.0%), followed by a nasal obstruction (65.0%) and headache. Patients with inflammatory and non-neoplastic lesions presented with headache (72.3%) and features of sinusitis (44.6%) [Table 1].
Table 1: Signs and symptoms at the time of presentation.
|
Unilateral nasal obstruction |
308 |
83 |
12 |
|
Epistaxis/bloody discharge |
21 |
98 |
4 |
|
Nasal mass |
24 |
28 |
8 |
|
Sinusitis |
193 |
9 |
21 |
|
Bilateral nasal obstruction |
11 |
35 |
4 |
|
Headache |
28 |
84 |
34 |
|
Diplopia |
7 |
17 |
0 |
|
Facial numbness |
7 |
75 |
7 |
|
Facial swelling/ deformity |
5 |
42 |
2 |
|
Anosmia |
5 |
18 |
1 |
|
Proptosis |
0 |
6 |
0 |
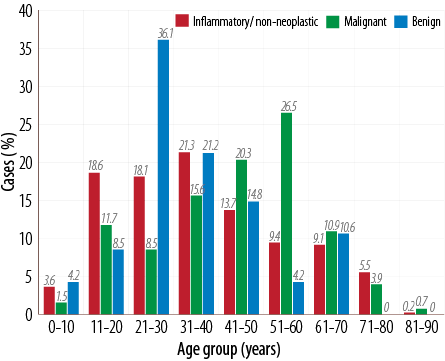
Figure 1: Age distribution of sinonasal tumors (n=610).
Nasal polyps were the most common benign tumors (67.6%) followed by Schneiderian papillomas and hemangiomas (n=29, 6.6%) [Table 2]. The most common overall site of involvement of all benign sinonasal lesions was the maxillary sinus (62.0%) followed by the frontal sinus and the right lateral nasal wall. Many of these tumors had more than one site of involvement. Nasopharyngeal carcinoma (NPC) was the most common malignant tumor. Out of the 52 cases diagnosed as NPC, 37 were squamous cell carcinomas (WHO Grade I), nine were non-keratinizing squamous carcinomas (WHO grade II), and six were lymphoepithelioma type carcinomas (WHO grade III). Small round cell tumors constituted a big category of malignant sinonasal tumors (n=21, 16.4%). Eight of these cases showed positivity for Pan-CK and were diagnosed as small cell carcinomas, seven of 21 cases were diagnosed as extraskeletal Ewing’s sarcoma and showed CD99 positivity. The other round cell tumors, after IHC panel, were two cases each of lymphoma (LCA, CD20), neuroblastoma (NSE), and rhabdomyosarcoma (Desmin). All cases of lymphoma were CD20 positive, CD5, CD15, and CD30 negative and the patients were diagnosed with diffuse large B-cell lymphoma [Table 3]. Fungal sinusitis was the most common type of inflammatory lesion comprising 63.0% of all inflammatory lesions.
Table 2: Spectrum of benign sinonasal tumors (n=435).
|
Nasal polyp |
294 (67.6) |
46 |
1.6:1 |
|
Schneiderian papillomas |
31 (7.1) |
|
|
|
Squamous |
14 (3.2) |
41.7 |
3.6:1 |
|
Oncocytoma |
1 (0.2) |
40 |
M |
|
Transitional |
1 (0.2) |
85 |
M |
|
Inverted |
15 (3.4) |
53 |
6.5:1 |
|
Hemangioma |
29 (6.7) |
31.8 |
1.2:1 |
|
Juvenile nasopharyngeal angiofibroma |
24 (5.5) |
16.5 |
M |
|
Fibrous dysplasia |
5 (1.1) |
21.2 |
2:3 |
|
Osteoma |
3 (0.7) |
28 |
M |
|
Cementifying fibroma |
3 (0.7) |
14 |
2:1 |
|
Giant cell tumor |
4 (0.9) |
18.5 |
1:1 |
|
Pleomorphic adenoma |
3 (0.7) |
42 |
1:2 |
|
Neurofibroma |
1 (0.2) |
45 |
M |
|
Schwannoma |
3 (0.7) |
59.3 |
M |
|
Low-grade glioma/brain herniation |
2 (0.5) |
27 |
M |
|
Paraganglioma |
1 (0.2) |
67 |
M |
* Data presented as n (%).
Table 3: Spectrum of malignant tumors of the sinonasal tract (n=128).
|
Nasopharyngeal carcinoma/squamous cell |
52 (40.6) |
45.6 |
1.2:1 |
|
Sinonasal undifferentiated |
17 (13.3) |
42 |
0.6:1 |
|
Small round cell |
21 (16.4) |
24.2 |
1.5:1 |
|
Non-Hodgkins lymphoma |
12 (9.4) |
49 |
1.4:1 |
|
Adenoid cystic |
10 (7.8) |
54.3 |
1.5:1 |
|
Sarcoma |
7 (5.5) |
52.4 |
2.5:1 |
|
Adenocarcinoma |
2 (1.6) |
52.5 |
1:1 |
|
Neuroendocrinal |
4 (3.1) |
30 |
1:3 |
|
Mucoepidermoid |
1 (0.8) |
57 |
M |
|
Melanoma |
1 (0.8) |
35 |
F |
* Data presented as n (%).
Discussion
Patients with sinonasal tumors presented mainly in the fourth decade of life. The lesion was benign in more than two-thirds and inflammatory in less than one-tenth of cases: the remaining being malignant. Male preponderance was observed in benign lesions while the malignant and inflammatory lesions were gender neutral. The most common symptom at presentation was unilateral nasal obstruction while an initial clinical diagnosis of chronic sinusitis was made in nearly one third of cases. Unilateral or bilateral nasal obstruction, blood-tinged nasal discharge, anosmia, diplopia, facial numbness, and facial swellings were the clinical features that favored a malignant sinonasal tumor. The maxillary sinus was the most common site of involvement in all lesions. CT and MRI scans were utilized to delineate tumor extent and bone destruction. Management was based on the anatomical site of origin and its extent, and histopathologic diagnosis. Even benign tumors had a tendency for local recurrence and bone destruction.
Nasal polyps are the most common benign tumors of the nasal cavity, as was seen in our study. They result from chronic inflammation, allergens, pollutants, infectious agents, chronic inflammation, and cystic fibrosis. There is no particular age or sex predilection.4,5 In our study, males were slightly more affected. For imaging, coronal sinus CT and endoscopy were used and for treatment NSAIDs, corticosteroids, and endoscopy-guided polypectomy.
Papillomas and inverted papillomas tend to be multicentric in up to 30% of cases and 5–20% of cases undergo malignant transformation.6,7 Papillomas in our study group were unicentric, and none of them showed bony erosion or reported malignant transformation during the study period. Complete resection using endoscopic approaches (n=20) or open surgery (n=11), for example, lateral rhinotomy with medial maxillectomy, were performed. The common risks involved with endoscopic sinus surgery including bleeding, infection, injury to the eye and its adnexa, cerebrospinal fluid (CSF) leak, and anosmia.8 We encountered anosmia and CSF leak in two cases each.
Hemangiomas are benign neoplasms of vascular origin with a marked male preponderance (approximately 6:1). Most cases in our study were capillary hemangiomas with almost no sex predilection. Lobular capillary hemangioma (LCH) was exclusively observed in female patients (n=12), mostly on the anteroinferior part of nasal septum (Little’s area) followed by anterior side of inferior turbinate (n=3). The most important underlying causes for LCH are a hormonal imbalance and excessive inflammatory response after local trauma. Endoscopic surgery, local cautery, electrocoagulation, cryotherapy and laser therapy are the various treatment modalities.8 There were no recurrences in any of our patients.
Juvenile nasopharyngeal angiofibroma (JNA) is seen in prepubertal and adolescent males. The average age of presentation in our study was 16.5 years, which was similar to another study where the average age was 17 years.9 Treatment with intranasal endoscopic surgery with/without flutamide (a testosterone receptor blocker) was used. Preoperative angiography in all cases showed the main blood supply from the internal maxillary artery. Embolization was used in eight cases (33.3%) to minimize blood loss.
Fibrous dysplasia is a relatively uncommon condition in the sinonasal tract and is characterized by replacement of normal bone by fibro-osseous connective tissue exhibiting varying degrees of metaplasia. Three of our patients were asymptomatic and two presented with a headache and hyposmia. CT scan showed ground glass opacity of inferior turbinate.
Osteomas are slow growing and mostly asymptomatic bony outgrowths and are well visualized in plain X-ray. All three cases in our study were seen in the frontoethmoid region. The tumors were removed surgically, and none of the patients reported recurrence. The other uncommon benign cementum-containing lesions were cementifying or ossifying fibromas. All patients (n=3) complained of dull pain and bone deformity. Lesions were radiolucent with few wispy trabeculae. These tumors do not usually recur after surgical resection.
Giant cell tumors (GCTs) of the head and neck are extremely rare and constitute 2% of all GCTs.10 Histopathology remains the gold standard for the diagnosis of these lesions. Aneurysmal bone cysts and giant cell reparative granuloma are the other differential diagnoses. These appeared multilocular on CT with multiple fluid levels. Treatment is aggressive surgery followed by radiotherapy. Pleomorphic adenomas originated from the intranasal septum or lateral nasal wall in all three cases and were intranasal as also observed in another study.11 Wide local excision via a lateral rhinotomy approach is the treatment of choice. Peripheral nerve sheath tumors namely schwannoma (n=3) and neurofibroma (n=1) are uncommonly encountered in the nasal cavity and paranasal sinuses. These tumors were treated with conservative surgical resection and had an excellent prognosis.
NPCs, particularly squamous cell carcinomas, are malignant tumors of the nasopharynx with an Ebstein Barr Virus (EBV) association. While rare in most parts of the world, it is endemic in Southern China, Southeast Asia, North Africa, and Arctica.12 There was a slight male preponderance in our study with a male to female ratio of 1.3:1. Although seen mostly in the fourth and fifth decades of life, they have been reported in patients as young as 22 years old.13 The most common presenting complaints were nasal bleeding, obstruction, headache and cervical lymphadenopathy. CT/MRI were done to determine the extent of the tumor and cervical lymphadenopathy. All tumors were treated with chemotherapy and radiotherapy. A study by Sanghvi et al,14 revealed a significant decrease in the annual incidence from 1973 to 2009 for the overall population; however, no significant differences were found (p>0.050) when comparing survival in the last three decades.
Sinonasal undifferentiated carcinoma (SNUC) has an aggressive clinical course and present with an advanced stage neoplasm. Eight out of 14 SNUCs in our study presented at stage T3 or T4 similar to a study by Gallo et al.15 Patients presented with nasal obstruction, epistaxis, proptosis, and facial pain. The average age at presentation was 42 years and the male to female ratio was 1:2. CT/MRI showed extensive bony destruction with the irregularity of margin of the tumor. The tumor was treated surgically with an arm of tumor-free surgical margins and orbital exenteration. Although described as a rare group, these were 11% in our study. The incidence may be attributed to various genetic and environmental factors.
Small round cell tumors (SRCT) constitute a heterogeneous group of malignant neoplasms characterized by a monotonous population of undifferentiated cells with relatively small sized hyperchromatic nuclei and scant cytoplasm. A definite diagnosis is usually not possible because of the frequent absence of distinguishing features or small biopsy size. Immunohistochemistry is mandatory in such cases using a panel of antibodies (CK, CD99, LCA, EMA, desmin, and vimentin). In our study, the majority were poorly differentiated carcinomas (8/20), followed by six lymphomas and three Ewings Sarcoma. However, in a study by Ashraf et al,16 mucosal melanomas were the most common SRCT.
Lymphomas of the sinonasal tract are uncommon malignancies representing 3–5% of all malignancies; non-Hodgkins lymphoma (NHL) accounts for 60% of all lymphomas. In the study by Chalastras et al,17 the most common was the diffuse large B-cell lymphoma; however, we exclusively encountered this subtype (12/12) in our study. In contrast, a study in Japan reported angiocentric lymphoma (35.9%) followed by B-cell lymphoma (22.6%) as the predominant histological types in Japan.18 The epidemiological difference may be attributed to the difference of genetic and environmental influences.
Adenoid cystic carcinoma is the second most common cancer occurring in the sinonasal tract and usually invades the lateral wall of the nose.19 However, it constituted less than 8% of all the malignant tumors we encountered. Although there is the high propensity of distant metastasis to lungs, we encountered metastasis to lungs in 50% of cases. All cases were subjected to combined primary excision with postoperative radiotherapy.
Almost all the patients who were exhibiting fungal sinusitis in our study were immunocompetent as also seen in the study by Hussain et al.20
Conclusion
Sinonasal tumors are complex, diverse and uncommon lesions that account for less than 3% of all tumors. Nearly a quarter of all sinonasal tumors are malignant as seen in our study with overlapping demographic and clinical features. The complex regional anatomy, proximity to vital structures, and delayed presentation following nonspecific symptoms make an accurate histopathologic diagnosis a mandatory prerequisite for planning effective management. The histologic type and grade of the tumor are representative of the biological behavior and the chemo- and radio-sensitivity of the mass and hence has an impeccable impact on its management. In short, histopathological diagnosis has prognostic value in sinonasal tumors.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- Pilch BZ, Bouquot J, Thompson LD. Squamous cell carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. (Kleihues P, Sobin LH, series editors). World Health Organization classification of tumours. Lyon, France: IARC Press; 2005. p. 15–17.
- Hopkin N, McNicoll W, Dalley VM, Shaw HJ. Cancer of the paranasal sinuses and nasal cavities. Part I. Clinical features. J Laryngol Otol 1984 Jun;98(6):585-595.
- McNicoll W, Hopkin N, Dalley VM, Shaw HJ. Cancer of the paranasal sinuses and nasal cavities. Part II. Results of treatment. J Laryngol Otol 1984 Jul;98(7):707-718.
- Lund VJ. Diagnosis and treatment of nasal polyps. BMJ 1995 Nov;311(7017):1411-1414.
- Lathi A, Syed MM, Kalakoti P, Qutub D, Kishve SP. Clinico-pathological profile of sinonasal masses: a study from a tertiary care hospital of India. Acta Otorhinolaryngol Ital 2011 Dec;31(6):372-377.
- Mansell NJ, Bates GJ. The inverted Schneiderian papilloma: a review and literature report of 43 new cases. Rhinology 2000 Sep;38(3):97-101.
- Al-Mujaini A, Wali U, Alkhabori M. Functional endoscopic sinus surgery: indications and complications in the ophthalmic field. Oman Med J 2009 Apr;24(2):70-80.
- Mills SE, Cooper PH, Fechner RE. Lobular capillary hemangioma: the underlying lesion of pyogenic granuloma. A study of 73 cases from the oral and nasal mucous membranes. Am J Surg Pathol 1980 Oct;4(5):470-479.
- Tang IP, Shashinder S, Gopala Krishnan G, Narayanan P. Juvenile nasopharyngeal angiofibroma in a tertiary centre: ten-year experience. Singapore Med J 2009 Mar;50(3):261-264.
- Bertoni F, Unni KK, Beabout JW, Ebersold MJ. Giant cell tumor of the skull. Cancer 1992 Sep;70(5):1124-1132.
- Compagno J, Wong RT. Intranasal mixed tumors (pleomorphic adenomas): a clinicopathologic study of 40 cases. Am J Clin Pathol 1977 Aug;68(2):213-218.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010 Dec;127(12):2893-2917.
- Al-Zaabi K, Al Riyami M, Al-Abri R. A unilateral maxillary sinus tumor. Oman Med J 2013 May;28(3):220-221.
- Sanghvi S, Khan MN, Patel NR, Yeldandi S, Baredes S, Eloy JA. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope 2014 Jan;124(1):76-83.
- Gallo O, Graziani P, Fini-Storchi O. Undifferentiated carcinoma of the nose and paranasal sinuses. An immunohistochemical and clinical study. Ear Nose Throat J 1993 Sep;72(9):588-590, 593-595.
- J Ashraf M, Beigomi L, Azarpira N, Geramizadeh B, Khademi B, Hakimzadeh A, et al. The small round blue cell tumors of the sinonasal area: histological and immunohistochemical findings. Iran Red Crescent Med J 2013 Jun;15(6):455-461.
- Chalastras T, Elefteriadou A, Giotakis J, Soulandikas K, Korres S, Ferekidis E, et al. Non-Hodgkin’s lymphoma of nasal cavity and paranasal sinuses. A clinicopathological and immunohistochemical study. Acta Otorhinolaryngol Ital 2007 Feb;27(1):6-9.
- Hatta C, Ogasawara H, Okita J, Kubota A, Ishida M, Sakagami M. Non-Hodgkin’s malignant lymphoma of the sinonasal tract–treatment outcome for 53 patients according to REAL classification. Auris Nasus Larynx 2001 Jan;28(1):55-60.
- Ko YH, Lee MA, Hong YS, Lee KS, Jung CK, Kim YS, et al. Prognostic factors affecting the clinical outcome of adenoid cystic carcinoma of the head and neck. Jpn J Clin Oncol 2007 Nov;37(11):805-811.
- Hussain S, Salahuddin N, Ahmad I, Salahuddin I, Jooma R. Rhinocerebral invasive myco-sis: occurrence in immunocompetent individuals. Eur J Radiol 1995 Jul;20(2):151-155.