Parathyroid tumors can be investigated in three groups; parathyroid adenoma (PA), atypical parathyroid adenoma (APA), and parathyroid carcinoma (PC). PC is the least common of these tumors and makes up less than 1% of all parathyroid tumors.1-5 The incidence is higher in Japan and Italy.4,6,7 This regional difference is not fully understood, but it is thought to be due to geographic differences or the use of different diagnostic criteria.1,2,8 PC cases clinically present with symptoms related to increased calcium levels in the blood due to invasive/metastatic carcinoma and palpable masses at the anterior region of the neck.2,3,5 Definite diagnostic criteria have been identified for the diagnosis of PC (e.g., metastasis, perineural invasion, capsular/vascular invasion). However, some cases present diagnostic difficulties and auxiliary diagnostic criteria have been identified. These include nuclear atypia, thick fibrous bands in the parenchyma, trabecular growth pattern, and increased mitotic activity. These are commonly found in PCs but can also be seen in benign parathyroid tumors.8-10
The term APA can be used for intermediate cases that did not demonstrate the essential criterias of malignancy, but those auxiliary diagnostic criterias were conspicuous. The search for more objective markers instead of subjective diagnostic criterias continues for the differential diagnosis of these lesions and immunohistochemistry (IHC) markers as well as molecular techniques are used in this regard.
The Ki-67 proliferation index is used for the diagnosis and differential diagnosis of many tumors. Although it is known to be higher in PCs than adenomas, no cut-off value providing a definite benign-malignant differentiation has been identified in parathyroid tumors.11,12 The World Health Organization (WHO) guide indicates that the malignancy risk is increased in cases with a Ki-67 proliferation index over 5%, and these cases should be followed-up closely.9,13
Galectin-3 is an animal lectin associated with tumor adhesion, angiogenesis, progression, and metastasis and binds to β-galactoside. Galectin-3 has been reported to play a role in tumorigenesis in thyroid carcinomas, colorectal carcinomas, breast carcinomas, brain tumors, melanomas, hepatocellular carcinomas, stomach carcinomas, esophagus carcinomas, and large cell lymphomas.14-16 Galectin-3 positivity indicates the resistance of the tumor or its ability to evade apoptosis. Galectin-3 is thought to contribute to the differential diagnosis of benign and malignant parathyroid tumors.11,14,17
Parafibromin is a protein coded by the CDC73/HRPT2 gene and consists of 531 amino acids. It serves in the regulation of cell proliferation, transcription, and histone modification and helps the human polymerase related factor 1 (PAF-1) complex related RNA polymerase II.3,4,18 CDC73/HRPT2 tumor suppressor gene inactivity is associated with the hereditary hyperparathyroidism-jaw tumor syndrome (HPT-JT) and sporadic parathyroid carcinomas.3,4,19
HBME-1 is a frequently used and valuable immunohistochemical marker. Recently it was used for the differential diagnosis of thyroid malignancies and had a reported sensitivity of 77% and specificity of 83% in malign thyroid tumors.20 In our study, HBME-1 was applied to all parathyroid tumors to assess the diagnostic utility in the differential diagnosis of parathyroid and thyroid neoplasms that sometimes could be confusing because of the very close adjacency of thyroid and parathyroid tissues.
We aimed to improve the differential diagnostic markers in parathyroid tumors by evaluating the use of parafibromin, Ki-67, galectin-3, and HBME-1.
Methods
Around 150 parathyroid lesions diagnosed at two different centers (Sifa University Pathology Department and Private Ege Pathology Laboratory) between 2006 and 2012 were re-evaluated. The study included 92 cases of which 84 were diagnosed with PA, six with APA, and two with PC according to their morphological, clinical, and radiological characteristics. Morphological characteristics included the presence of normal parathyroid tissue, capsule/vascular invasion, thick capsule, broad fibrous bands, trabecular growth pattern, macronucleoli, mitosis, and necrosis. Clinical characteristics considered the parathyroid hormone (PTH) level measured after tumor excision during frozen section. Capsule invasion was present in one case diagnosed with PC and in another case capsule/vascular invasion was present. The most significant characteristic feature of APA cases was the thick capsule, which seemed to limit the tumor, broad fibrous bands, and trabecular growth pattern. In spite of these striking features, no capsule and/or vascular invasion was observed in these tumors. There were no definite malignancy criteria or other auxiliary morpholological criteria in the PA cases.
The first patient with PC had been operated previously for breast carcinoma and received medical treatment. During routine follow-up, elevated calcium was reported, and a mass lesion was found in the parathyroid region. The patient was diagnosed with PC postoperatively and followed-up. Widespread multiorgan metastasis was found two years after the PC was diagnosed. The case was lost due to widespread metastases four years after the PC diagnosis. Another patient was diagnosed with PC and lung metastasis was found six months later. The serum calcium level was measured to be very high and could not be controlled. The patient was hospitalized and treated for a long time. The patient is still alive and is stable. There was no family history of parathyroid carcinoma in either case.
No sign of recurrence and/or metastasis was found in the patients diagnosed with PA or APA that we could contact.
We prepared 4 µm sections of all formalin-fixed and paraffin-embedded tissues of the 92 cases on positively charged slides for galectin-3 (NCL-GAL3, clone 9C4, dilution 1:100; NovoCastra, Germany), Ki-67 (clone MIB-1, dilution 1:100; Dako, Denmark) and HBME-1 (monoclonal anti-mesothelial cell, clone HBME-1, dilution 1:50; Dako. Denmark) immunostaining. For parafibromin (HRPT2, dilution 1/100, Santa Cruz Biotechnology, USA) staining, 1 mm microarray tissues were obtained from all cases and 4 µm sections were similarly placed on positively charged slides. All immunohistochemical staining procedures including deparaffinization and antigen retrieval were performed on the Dako LV-1 automated immunostainer. The assessment criteria and cut-off values of former studies were taken into consideration in the evaluation of staining features and percentages of parafibromin, galectin-3, and Ki-67.11,12,14,16,17 Nuclear parafibromin positivity in tumor cells was accepted as positive staining. While assessing nuclear positivity, we ignored staining intensity and the percentage of positive tumor cells because of the small amount of tumor tissue by the microarray method. Cytoplasmic and membranous staining was considered positivity for galectin-3 staining. The galectin-3 staining pattern was accepted as 3+ (>60% of the tumor cell positive), 2+ (31–60% of the tumor cell positive), 1+ (<30% of the tumor cell positive), and negative (no reaction).17 Nuclear Ki-67 staining was considered to be positive. The most intense Ki-67-stained area in the tissue was evaluated and scored as under 1%, 1–5%, and over 5%.11,14 Positive cytoplasmic and membranous staining with HBME-1 was accepted as positive, and a 30% or less extent of positive staining was considered focally positive whereas staining of more than 30% was considered diffuse.
SPSS Statistics (SPSS Inc., Chicago, US) version 16 was used for statistical analyzes. In addition to descriptive statistical methods, the chi-square test and Fisher’s exact test were used to compare the categorical variables. A p-value <0.050 was considered statistically significant.
Results
Of the 92 cases included in the study, 84 were diagnosed with PA, six with APA, and two with PC. There were 82 females and 10 males. Parathyroid tumors were 8.2 times more common in females [Table 1]. The mean age of patients was 50.9 years (min: 23 years, max: 83 years), and the mean tumor diameter was 1.97 cm (min: 1.0 cm, max: 4.5 cm). No statistical relation was found between tumor type and gender, age distribution, and tumor diameter.
Table 1: Clinico pathological and immunohistochemical features of patients with parathyroid tumors.
|
Gender |
|
|
|
|
|
|
Male |
1 |
2 |
7 |
10 |
10.8 |
|
Female |
1 |
4 |
77 |
82 |
89.2 |
|
Parafibromin |
|
|
|
|
|
|
Positive |
0 |
6 |
84 |
90 |
97.8 |
|
Negative |
2 |
0 |
0 |
2 |
2.2 |
|
Galectin-3 |
|
|
|
|
|
|
3+ |
1 |
0 |
4 |
5 |
5.4 |
|
2+ |
0 |
0 |
4 |
4 |
4.4 |
|
1+ |
1 |
3 |
9 |
13 |
14.1 |
|
Negative |
0 |
3 |
67 |
70 |
76.1 |
|
Ki-67 |
|
|
|
|
|
|
<1% |
0 |
5 |
72 |
75 |
81.5 |
|
1-5% |
2 |
0 |
12 |
15 |
16.3 |
|
>5% |
0 |
1 |
1 |
2 |
2.2 |
|
HMBE-1 |
|
|
|
|
|
|
>30% |
0 |
0 |
10 |
10 |
10.8 |
|
<30% |
1 |
0 |
16 |
17 |
18.5 |
|
Negative |
1 |
6 |
58 |
65 |
70.7 |
|
Latest health status |
|
|
|
|
|
|
Alive (84 PA, 6 APA, 1 PC) |
1 |
6 |
84 |
91 |
98.9 |
PC: parathyroid carcinoma; PA: parathyroid adenoma; APA: atypical parathyroid adenoma.
In typical tumors that had PA features morphologically, positivity was demonstrated with parafibromin and diffuse positivity with galectin-3 and HBME-1 immunohistochemically [Figure 1]. In PCs that exceeded the capsule and invaded the adjacent tissues parafibromin was found to be negative whereas galectin-3 and HBME-1 were focally positive immunohistochemically [Figure 2]. The main IHC features of all tumor groups are summarized in Table 1. Parafibromin was negative in two PC cases, but positive in all APA and PA cases. Positivity was observed with galectin-3 in 17 PA cases, three APA cases, and two PC cases. HBME-1 was positive in 26 PA cases and one PC case. A statistically significant difference was present between the PA, APA and PC groups in parafibromin and galectin-3 expression (p=0.000 and p=0.000, respectively), but not for HBME-1 expression (p=0.480) [Table 2]. A statistically significant difference was found between the three tumor groups according to the Ki-67 score (p=0.010) [Table 2].
Higher parafibromin expression, less galectin-3 positivity, and a low Ki-67 proliferation index (under 1%) was found in the PA cases compared to PC and APA cases.
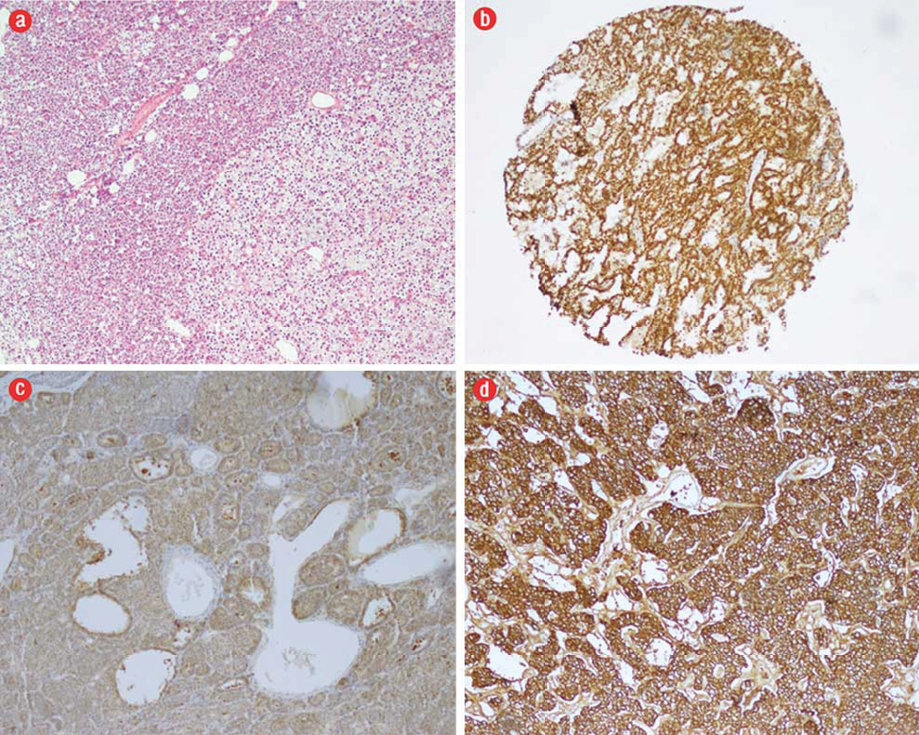
Figure 1: (a) Areas morphologically compatible with parathyroid adenoma (PA) (hematoxylin and eosin, magnification = 200 ×). (b) Nuclear parafibromin positivity in tissues prepared with the microarray method, magnification = 20 ×. (c) Diffuse galectin-3 positivity in PA, magnification = 10 ×. (d) Diffuse HBME-1 positivity was seen in some PA cases, magnification = 20 ×.
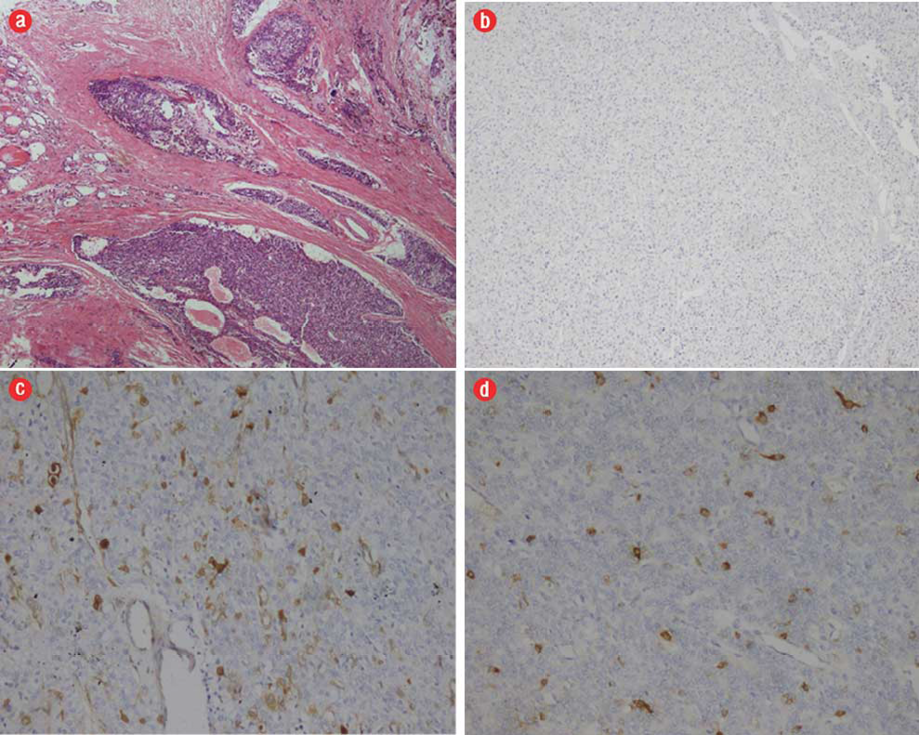
Figure 2: (a) Hematoxylin and eosin staining. Infiltration of thyroid and other adjacent tissues with tumor together with tumor cells in vascular spaces in parathuroid carcinoma (PC), magnification = 5 ×. (b) Parafibromin was negative in PC, magnification = 40 ×. (c) Galectin-3 was focally positive in PC, magnification = 40 ×. (d) HBME-1 was focally positive in PC, magnification = 40 ×.
Table 2: The relationship between tumor type immunohistochemical parameters.
|
Parafibromin |
|
|
|
0.000 |
|
Positive |
84 |
6 |
0 |
|
|
Negative |
0 |
0 |
2 |
|
|
Galectin-3 |
|
|
|
0.000 |
|
3+ |
4 |
0 |
1 |
|
|
2+ |
4 |
0 |
0 |
|
|
1+ |
9 |
3 |
1 |
|
|
Negative |
67 |
3 |
0 |
|
|
Ki-67 |
|
|
|
0.010 |
|
>1% |
72 |
5 |
0 |
|
|
1–5% |
12 |
0 |
2 |
|
PC: parathyroid carcinoma; PA: parathyroid adenoma; APA: atypical parathyroid adenoma.
Discussion
Our data indicate that parafibromin expression loss and galectin-3 expression could help the differential diagnosis of parathyroid tumors. The parafibromin expression loss observed only in PC cases is consistent with the literature. In a study conducted by Tan et al,4 the sensitivity and specificity of parafibromin nuclear immunoreactivity in PCs was reported as 96% and 99%, respectively. Parafibromin nuclear positivity was reported to potentially disappear in adenomas associated with the HPT-JT syndrome. This is rare in sporadic PA.4,11,21-24
Although only two carcinoma cases were present in our study, parafibromin was negative in both and positive in all PA and APA cases. The presence of parafibromin expression loss only in PC cases could contribute to the differential diagnosis in light of the literature data and our results. Moreover, parafibromin use as a potential indicator of tumor aggressiveness has been studied in colon, lung, and gastric malignancies.25-27 The role and importance of galectin-3 in the differential diagnosis of parathyroid tumors have been proven with many studies.11,14,17,23 Bergero et al,14 reported 92.3% galectin-3 positivity in 26 PCs. In the same study, positivity was seen in only one PA case, but no information was given on whether it was focal or diffuse. Focal (1+) galectin-3 positivity was found in 13 of 84 PAs in our study. Although there was a significant difference between the three groups in terms of galectin-3 expression, it should be kept in mind that focal galectin-3 positivity may be present in PC cases as well as PA and APA cases.
No information about HBME-1 expression in parathyroid tumors was found in the literature. We found HBME-1 positivity in 30.9% of PA cases and one PC case. The presence of positivity in adenomas is thought to be useful for the differential diagnosis, but we believe that studies on larger series would be appropriate.
A high Ki-67 proliferation index could aid the differential diagnosis of malign and benign tumors. The sensitivity and specificity of the Ki-67 proliferation index in parathyroid tumors has been reported as 60% and 93.3–100%, respectively.11,14 The Ki-67 cut-off value was accepted as 5% in certain studies.11 Ki-67 was found to be under 5% in most of our cases (including PC and APA), but over 5% only in three cases (two PA and one APA). Although no clear Ki-67 cut-off value that would ensure the differentiation was found in our study, the fact that it was mostly less than 1% in PAs is striking.
Conclusion
The number of PCs in our series was small so our data mostly reflects the immunohistochemical characteristics of PAs. The results have shown that continued parafibromin expression is suggestive of PA diagnosis. Galectin-3 expression can also be helpful in the differential diagnosis of PA and PC. Additionally, a Ki-67 proliferation index under 1% can contribute to the diagnosis of PA.
Pathologists and surgeons know the examination of parathyroid tumors with frozen section can be troublesome and unfeasible. During surgery, the clinical data given by the surgeon such as the cohesiveness of tumor to peripheral tissues can be helpful in assessment. But, after surgery the pathological diagnosis is mostly based on the morphological and immunohistochemistry examination.
Although the PC diagnosis is a rare, situation examining parafibromin, galectin-3, and the Ki-67 proliferation index in the parathyroid immunohistochemically can contribute to the differential diagnosis.
Disclosure
The authors declared no conflicts of interest. No funding was recieved for this study.
references
- Grimelius L, Johansson H. Pathology of parathyroid tumors. Semin Surg Oncol 1997 Mar-Apr;13(2):142-154.
- Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab 2001 Feb;86(2):485-493.
- Juhlin CC, Villablanca A, Sandelin K, Haglund F, Nordenström J, Forsberg L, et al. Parafibromin immunoreactivity: its use as an additional diagnostic marker for parathyroid tumor classification. Endocr Relat Cancer 2007 Jun;14(2):501-512.
- Tan MH, Morrison C, Wang P, Yang X, Haven CJ, Zhang C, et al. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clin Cancer Res 2004 Oct;10(19):6629-6637.
- Mittendorf EA, McHenry CR. Parathyroid carcinoma. J Surg Oncol 2005 Mar;89(3):136-142.
- Obara T, Okamoto T, Kanbe M, Iihara M. Functioning parathyroid carcinoma: clinicopathologic features and rational treatment. Semin Surg Oncol 1997 Mar-Apr;13(2):134-141.
- Favia G, Lumachi F, Polistina F, D’Amico DF. Parathyroid carcinoma: sixteen new cases and suggestions for correct management. World J Surg 1998 Dec;22(12):1225-1230.
- Haven CJ, van Puijenbroek M, Karperien M, Fleuren GJ, Morreau H. Differential expression of the calcium sensing receptor and combined loss of chromosomes 1q and 11q in parathyroid carcinoma. J Pathol 2004 Jan;202(1):86-94.
- Bondeson L, Sandelin K, Grimelius L. Histopathological variables and DNA cytometry in parathyroid carcinoma. Am J Surg Pathol 1993 Aug;17(8):820-829.
- Duan K, Mete Ö. Parathyroid Carcinoma: Diagnosis and Clinical Implications. Turk Patoloji Derg 2015;31(Suppl 1):80-97.
- Fernandez-Ranvier GG, Khanafshar E, Tacha D, Wong M, Kebebew E, Duh QY, et al. Defining a molecular phenotype for benign and malignant parathyroid tumors. Cancer 2009 Jan;115(2):334-344.
- Lloyd RV, Carney JA, Ferreiro JA, Jin L, Thompson GB, Van Heerden JA, et al. Immunohistochemical analysis of the cell cycle-associated antigens Ki-67 and retinoblastoma protein in parathyroid carcinomas and adenomas. Endocr Pathol 1995;6(4):279-287.
- DeLellis RA. Parathyroid carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2004. p. 124–127.
- Bergero N, De Pompa R, Sacerdote C, Gasparri G, Volante M, Bussolati G, et al. Galectin-3 expression in parathyroid carcinoma: immunohistochemical study of 26 cases. Hum Pathol 2005 Aug;36(8):908-914.
- Orlandi F, Saggiorato E, Pivano G, Puligheddu B, Termine A, Cappia S, et al. Galectin-3 is a presurgical marker of human thyroid carcinoma. Cancer Res 1998 Jul;58(14):3015-3020.
- Cay T. Immunhistochemical expression of galectin-3 in cancer: a review of the literature. Turk Patoloji Derg 2012;28(1):1-10.
- Saggiorato E, Bergero N, Volante M, Bacillo E, Rosas R, Gasparri G, et al. Galectin-3 and Ki-67 expression in multiglandular parathyroid lesions. Am J Clin Pathol 2006 Jul;126(1):59-66.
- Rozenblatt-Rosen O, Hughes CM, Nannepaga SJ, Shanmugam KS, Copeland TD, Guszczynski T, et al. The parafibromin tumor suppressor protein is part of a human Paf1 complex. Mol Cell Biol 2005 Jan;25(2):612-620.
- Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med 2003 Oct;349(18):1722-1729.
- de Matos LL, Del Giglio AB, Matsubayashi CO, de Lima Farah M, Del Giglio A, da Silva Pinhal MA. Expression of CK-19, galectin-3 and HBME-1 in the differentiation of thyroid lesions: systematic review and diagnostic meta-analysis. Diagn Pathol 2012;7:97.
- Juhlin C, Larsson C, Yakoleva T, Leibiger I, Leibiger B, Alimov A, et al. Loss of parafibromin expression in a subset of parathyroid adenomas. Endocr Relat Cancer 2006 Jun;13(2):509-523.
- Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet 2002 Dec;32(4):676-680.
- Truran PP, Johnson SJ, Bliss RD, Lennard TW, Aspinall SR. Parafibromin, galectin-3, PGP9.5, Ki67, and cyclin D1: using an immunohistochemical panel to aid in the diagnosis of parathyroid cancer. World J Surg 2014 Nov;38(11):2845-2854.
- Kruijff S, Sidhu SB, Sywak MS, Gill AJ, Delbridge LW. Negative parafibromin staining predicts malignant behavior in atypical parathyroid adenomas. Ann Surg Oncol 2014 Feb;21(2):426-433.
- Zheng HC, Wei ZL, Xu XY, Nie XC, Yang X, Takahashi H, et al. Parafibromin expression is an independent prognostic factor for colorectal carcinomas. Hum Pathol 2011 Aug;42(8):1089-1102.
- Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, et al. Downregulated parafibromin expression is a promising marker for pathogenesis, invasion, metastasis and prognosis of gastric carcinomas. Virchows Arch 2008 Feb;452(2):147-155.
- Xia P, Wang W, Xu XY, Wang JP, Takano Y, Zheng HC. Parafibromin expression in lung normal tissue and carcinoma: its comparison with clinicopathological parameters of carcinoma. Histol Histopathol 2011 Aug;26(8):1039-1047.