Kimura disease is a rare idiopathic inflammatory disease that presents as painless nodules and masses in the head and neck region. Its presentation as a large upper limb swelling is quite unusual and could be misdiagnosed as malignancy if one is not familiar with the entity.
Case Report
A 41-year-old man presented with a history of painless swelling in the right arm that gradually increased in size over a period of 28 years. He was concerned by the disfigurement and did not complain of any local symptoms like pruritus, functional compromise, or constitutional symptoms. Clinical examination revealed a firm, soft tissue mass measuring approximately 15cm×11cm on the medial aspect of the arm and elbow, with no neurovascular deficits. The overlying skin was unremarkable.
Laboratory tests revealed a high white blood count (WBC) of 20.70×109/L (reference range 3.40–9.60×109/L) with 62.7% eosinophils and a slightly elevated erythrocyte sedimentation rate (ESR). His renal panel was unremarkable (4.7mmol/L blood urea, 78µmol/L serum creatinine, 139mmol/L sodium, 4.3 mmol/L potassium, and >60mL/min estimated glomerular filtration rate). Serum immunoglobulin E levels were not obtained.
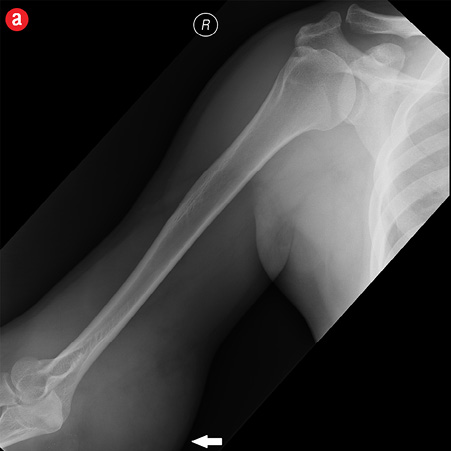
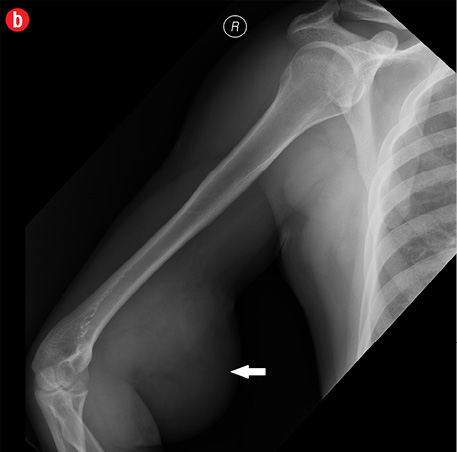
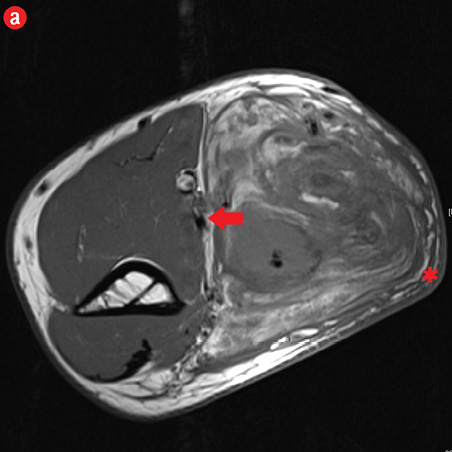
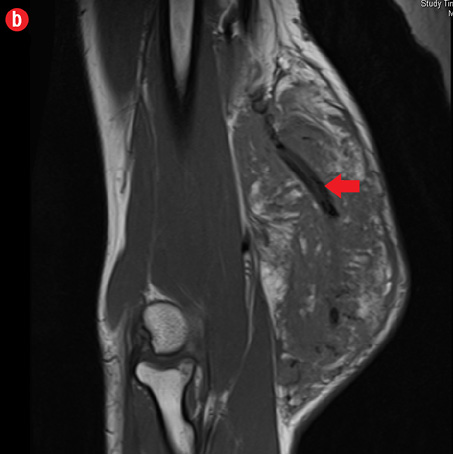
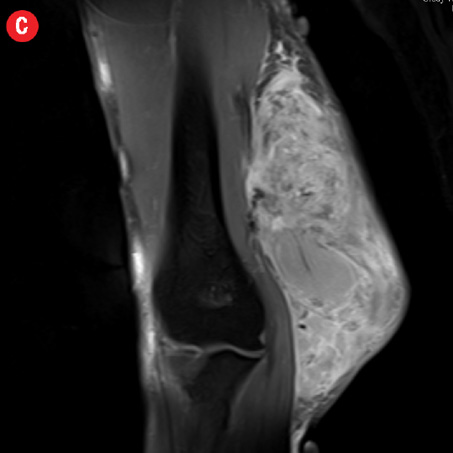
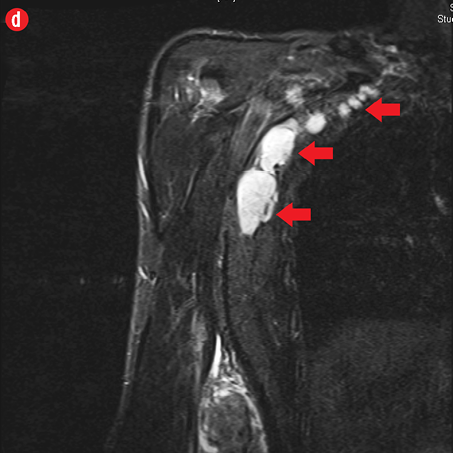
Initial radiographs [Figure 1a and 1b] showed a large soft tissue swelling on the medial aspect of the distal arm and elbow with no internal calcification. The underlying bone had no changes. Further characterization using magnetic resonance imaging (MRI) revealed a large subcutaneous soft tissue mass in the medial epitrochlear region, which appeared iso to hyperintense on T1- and hyperintense on T2-weighted images compared to muscle signals [Figure 2]. There was no necrosis, cystic degeneration, or calcifications identified within the mass. Adjacent muscles were displaced with no altered signals, suggesting a slow growing nature of the mass that was consistent with the long clinical history. There was mild fat stranding of the overlying skin. The brachial vessels were engulfed by the mass, which demonstrated moderate heterogeneous enhancement on contrast. Multiple axillary lymph nodes were enlarged and displayed signal characteristics and enhancement similar to the mass lesion.
|
|
|
|
|
Figure 1: A 41-year-old man with Kimura’s disease. (a) Frontal and (b) lateral radiograph of the arm and elbow joint showed a soft tissue swelling (white arrows) in the anterior and medial aspect of the distal arm and elbow. There was no internal calcification or changes in the underlying bones identified. |
|
|
|
|
|
|
|
|
|
Figure 2: A 41-year old man with Kimura disease. (a) Axial T1-weighted magnetic resonance imaging (MRI) of the distal arm showed a heterogeneous mass lesion in the subcutaneous plane of the medial aspect of the distal arm causing displacement of the underlying muscles and neurovascular brachial bundle (arrow). Note the subcutaneous fat stranding (asterisk). (b) Coronal T1-weighted MRI of the distal arm and elbow showed the vertical extent of the mass in the subcutaneous plane. Note the linear flow voids (arrow) inside the mass representing vascular structures. (c) Coronal fat suppressed post-contrast T1-weighted MRI of the distal arm and elbow showed abundant enhancement of the mass. No abnormal enhancement of the underlying muscle plane. (d) Fat suppressed coronal T2-weighted MRI of the distal arm and elbow showed the subcutaneous mass appearing predominantly hyperintense to muscles. Multiple enlarged axillary lymph nodes (arrows) also appeared hyperintense. |
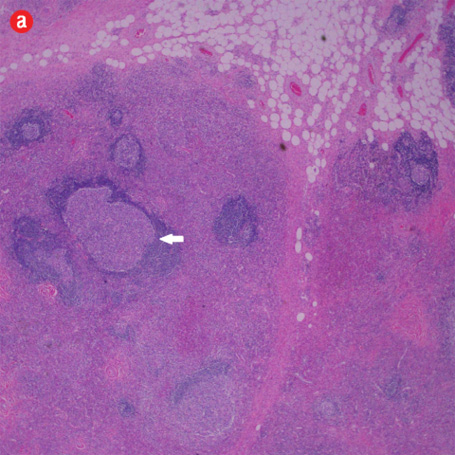
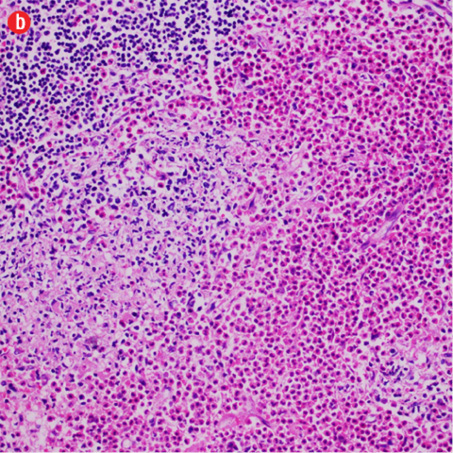
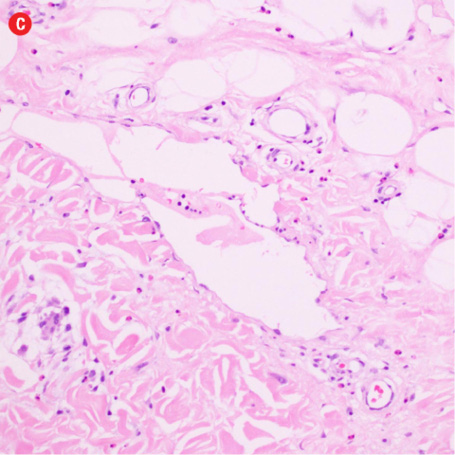
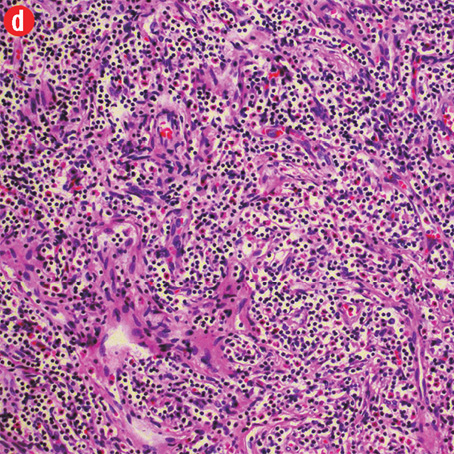
Based on the patient’s clinical, demographic, laboratory, and imaging features, Kimura disease was considered the most probable diagnosis. The patient opted for surgical excision in the view of cosmetic concerns and anxiety to know the exact pathology. Histopathological examination revealed large confluent lymphoid and eosinophil-rich areas with background stromal fibrosis [Figure 3]. Nodules of hyperplastic lymphoid follicles of various sizes accompanied by expanded germinal centers, numerous eosinophils, and increased vascularity were also evident. There were no anaplastic cells, Hodgkin, or Reed-Sternberg cells.
|
|
|
|
|
|
|
|
|
Figure 3: Histopathology revealed (a) near effacement of normal tissue by a dense infiltrate and some lymphoid aggregates with prominent germinal centers (arrow), magnification=40×, and (b) solid sheets of eosinophils and microabscess with necrosis, magnification=200×. (c) Hyalinized vessels and stroma were present at the periphery of the infiltrate, magnification=200×. (d) The proliferation of thin walled venules (arrows) associated with dense lymphoid and eosinophilic infiltrate was also seen, magnification=200×. The endothelial cells in Kimura’s disease are not plump or prominent. |
Overall, the histopathological picture was in accordance with the clinical and imaging picture of Kimura disease.
Discussion
Kimura disease is a rare benign idiopathic chronic inflammatory disease. Although its initial description was made in 1937, the disease started to be called by its present name only after Kimura and Ishikawa described its distinct pathological entity.1 The disease typically affects Asians in their second and third decades of life and has a male predominance [Table 1].2
Table 1: Summary of the features of Kimura’s disease.
|
Etiology |
Not clearly understood. An immunological response to a self-limiting allergic reaction elicited by an unknown stimulus is proposed. |
|
Gender ratio |
Male (87%): female (13%) |
|
Age predilection |
Second and third decades. |
|
Risk factors |
The Asian/oriental population is mainly affected. |
|
Treatment |
Controversial; options between multiple conservative treatment and surgical resection. Surgical excision is ideal for single circumscribed lesion. Combined medical and radiotherapy for recurrence and systemic involvement. |
|
Prognosis |
Good. No chance of turning to malignancy. |
The cause of the disease has not yet been identified. An immunological response to a self-limiting allergic reaction elicited by an unknown stimulus was proposed as substantiated by elevated peripheral blood eosinophils and serum immunoglobulin E.3 Histopathologically, the lesions are characterized by hyperplasia of lymphoid follicles, eosinophilic infiltrations and proliferation of fibrocollagenous tissue and post-capillary venules.4
The usual clinical presentation is a painless and slowly progressing subcutaneous nodules/masses, frequently affecting the head and neck region associated with regional lymphadenopathy. The duration of the swelling can range from one month to 25 years.5 In the case presented here, the patient had a history of swelling dating back to 28 years; the longest history to date in the literature. In the head and neck region, there is frequent involvement of major salivary glands, especially the parotid and periparotid regions.5,6 Other salivary glands, the lacrimal gland, oral cavity, and pharynx can also be affected. Other less common locations of the disease include the axilla, extremities, and abdomen. The masses are frequently multiple.5,6 Unilateral or bilateral lymphadenopathy is commonly seen.
On ultrasound, the lesions appeared solid and hypoechoic. The lymph nodes appeared round, well-defined, hypoechoic, and showed a preserved central fatty hilum. On Doppler, the lymph nodes showed a predominant hilar pattern of vascularity with decreased intranodal resistance.7 The subcutaneous masses are commonly ill-defined and isodense to hypodense on computed tomography (CT) scans with no necrosis or calcification.5,6,8 Diffuse atrophy of subcutaneous fat may be seen, reflecting the chronic and indolent nature of the disease.5 Contrast enhancement of the lesion can vary from mild to marked and homogeneous to heterogeneous, reflecting the varied amount of vascular proliferation and fibrotic component on histology. On follow-up, the lesions may show declining enhancement as the disease progresses, which is explained by progressive fibrosis and sclerosis.9 If the subcutaneous lesions are bilateral and multiple, all the lesions tend to show relatively uniform imaging characteristics in the same patient.5 On MRI, the lesions appeared iso to hyperintense on T1- and hyperintense on T2-weighted images. Contrast MRI showed a similar enhancement pattern as CT. The enlarged lymph nodes also often exhibit the same imaging and enhancement characteristics as the subcutaneous lesions in the same individual. Upper limb involvement of Kimura’s disease characteristically presents as a mass in the epitrochlear region adjacent to the major lymphatics and medial neurovascular bundle.10 Masses in the upper limb usually show multiple internal flow voids representing vascular structures, variable amount of subcutaneous edema, and produce mass effect over adjacent muscles and neurovascular structures without any signal changes in them.10
Differential diagnosis on imaging would include tuberculosis, lymphoma, metastatic adenopathy, primary parotid tumors, angiolymphoid hyperplasia with eosinophilia (ALHE), and cat-scratch disease [Table 2].7,10,11 In cases of upper limb disease, as in our case, soft tissue sarcomas can also be considered as differential. In tuberculosis, the lymph nodes show central necrosis and tendency to matting, the features that are not seen in Kimura’s disease. Lymphomatous enlargement of the nodes is often bilateral with eccentric cortical hypertrophy and no changes in the underlying subcutaneous fat. Primary parotid tumors are well defined and encapsulated, unlike Kimura’s disease. Metastatic lymph nodes may show internal necrosis and evidence of primary elsewhere. On ultrasound the metastatic nodes show loss of central fatty hilum and predominant capsular vascularity in contrast to Kimura’s disease.7 Cat-scratch disease can mimic Kimura’s disease on imaging as it can produce lymph node enlargement in the medial epitrochlear region and axilla with surrounding subcutaneous fat stranding. However, in the cat-scratch disease there is a history of exposure to cat, positive serology, and lymph nodes often show central necrosis. ALHE is a distinct entity where there is no sex preference, nodules are more superficial and smaller, and lymphadenopathy is uncommon. Histopathologically, the lesions of ALHE do not show elements of fibrosis and sclerosis, which are uniformly seen in all cases of Kimura’s disease regardless of its stage.
Table 2: Differential diagnoses of Kimura’s disease based on imaging.
|
Kimura’s disease |
Subcutaneous masses are solid and hypoechoic with the lymph nodes showing preserved central fatty hilum and predominant hilar pattern of vascularity. |
There are ill-defined solid masses in the head and neck especially around the major salivary glands often associated with regional adenopathy. Characteristic imaging findings include moderate to marked enhancement of the masses and nodes with no calcification or necrosis. |
|
Tuberculosis |
Lymph nodes demonstrate loss of fatty hilum, tendency for matting, and central necrosis. |
Calcifications are common. Central non-enhancing areas in the nodes reflect caseous necrosis. |
|
Lymphoma |
Nodes are pseudocystic in appearance with profuse central and peripheral vascularity and eccentric cortical hypertrophy. |
Nodes are commonly bilateral and bulkier with no changes in the underlying subcutaneous fat. |
|
Metastatic adenopathy |
Nodes show loss of central fatty hilum with a capsular pattern of vascularity. Necrosis and calcification may be seen. |
Evidence of primary mass lesion may be found elsewhere. |
|
Primary parotid tumors |
Parotid tumors are usually capsulated and may appear heterogeneous with cystic areas and calcification. |
Parotid tumors are well-circumscribed. Calcifications, necrosis, and cystic areas are commonly appreciated. |
|
Angiolymphoid hyperplasia with eosinophilia (ALHE) |
-- |
There is no male predominance. Nodules are more superficial and smaller, and lymphadenopathy is uncommon. |
|
Cat-scratch disease |
Nodes often show central necrosis. |
Clues to diagnosis include central necrosis of lymph nodes, history of exposure to cat, and positive serology. |
Management of Kimura’s disease is controversial and options are between surgical resection and various conservative treatments such as systemic steroids, cytotoxic drugs, and radiotherapy.12 Surgical excision is treatment of choice for single and well-defined lesion with advantage of precise histopathological confirmation. Conservative treatments are opted for recurrences and systemic involvement. Though considered benign, Kimura’s disease has a recurrence rate of 25–75% after surgical excision.13 The chance of recurrence is lower when two or more conservative treatments are combined.11
Complications of Kimura’s disease include nephrotic syndrome and proteinuria.14 Other minor allergic complications, such as asthma, urticaria, dermatitis, and rhinitis, are also reported.5 Malignant transformation is not known to occur.
Conclusion
Because of its rare occurrence and non-specific imaging findings, Kimura’s disease can be missed especially in non-endemic regions. Correlation with clinical history, laboratory parameters, and imaging appearances can aid in precise diagnosis, spare the patients from multiple invasive diagnostic procedures, and alleviate their anxiety.
Disclosure
The authors declared no conflicts of interest.
references
- Kimura T, Yoshimura S, Ishikawa E. Unusual granulation combined with hyperplastic changes of lymphatic tissue (in Japanese). Trans Soc Pathol Jpn 1948;37:179-180.
- Takagi K, Harada T, Ishikawa E. Kimura’s disease eosinophilic: lymphfolliculoid granuloma. Nihon Rinsho. 1993 Mar; 51(3):785-788.
- Shetty AK, Beaty MW, McGuirt WF Jr, Woods CR, Givner LB. Kimura’s disease: a diagnostic challenge. Pediatrics 2002 Sep;110(3):e39.
- Chen H, Thompson LD, Aguilera NS, Abbondanzo SL. Kimura disease: a clinicopathologic study of 21 cases. Am J SurgPathol. 2004 Apr; 28(4):505-513.
- Zhang R, Ban XH, Mo YX, Lv MM, Duan XH, Shen J, et al. Kimura’s disease: the CT and MRI characteristics in fifteen cases. Eur J Radiol. 2011 Nov; 80(2):489-497.
- Park SW, Kim HJ, Sung KJ, Lee JH, Park IS. Kimura disease: CT and MR imaging findings. AJNR Am J Neuroradiol. 2012 Apr; 33(4):784-788.
- Ahuja A, Ying M, Mok JS, Anil CM. Gray scale and power Doppler sonography in cases of Kimura disease. AJNR Am J Neuroradiol. 2001 Mar; 22(3):513-517.
- Gopinathan A, Tan TY. Kimura’s disease: imaging patterns on computed tomography. Clin Radiol 2009 Oct;64(10):994-999.
- Lim WE, Tan NG, Tan KP. Radiological features in a patient with Kimura’sdisease. Singapore Med J. 1997 Mar; 38(3):125-128.
- Choi JA, Lee GK, Kong KY, Hong SH, Suh JS, Ahn JM, et al. Imaging findings of Kimura’s disease in the soft tissue of the upper extremity. AJR Am J Roentgenol 2005 Jan;184(1):193-199.
- Hiwatashi A, Hasuo K, Shiina T, Ohga S, Hishiki Y, Fujii K, et al. Kimura’s disease with bilateral auricular masses. AJNR Am J Neuroradiol 1999 Nov-Dec;20(10):1976-1978.
- Takeishi M, Makino Y, Nishioka H, Miyawaki T, Kurihara K. Kimura disease: diagnostic imaging findings and surgical treatment. J Craniofac Surg. 2007 Sep; 18(5):1062-1067.
- Park JS, Jin W, Ryu KN, Won KY. Bilateral asymmetric superficial soft tissue masses with extensive involvement of both upper extremities: demonstration of Kimura’s disease by US and MRI (2008: 12b). Eur Radiol 2009 Mar;19(3):781-786.
- Rajpoot DK, Pahl M, Clark J. Nephrotic syndrome associated with Kimura disease. Pediatr Nephrol 2000 Jun;14(6):486-488.