Being a mature B cell malignancy, the finding of monoclonal proteins is not unexpected in chronic lymphocytic leukemia (CLL). Recent reports indicate that over 60% of patients have circulating monoclonal proteins.1 Biclonal gammopathy, defined as the simultaneous appearance of two different monoclonal protein components, is rare. It is thought to result from the production of two different components by a single clone or by two different malignant clones (developing simultaneously or one evolving from the other as a transformation event). Very few patients with CLL having biclonal gammopathies have been reported. We report the case of a patient with CLL who developed biclonal gammopathy with rapid progression and resistance to treatment.
Case report
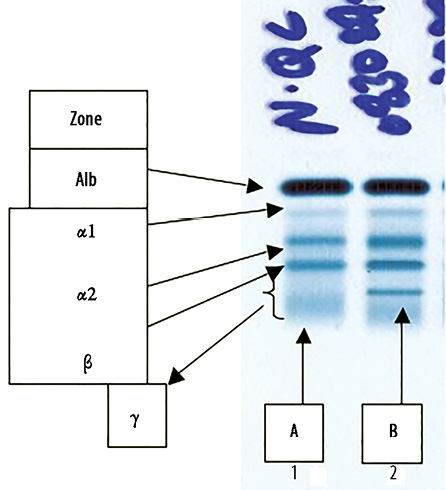
Figure 1: Serum protein electro-phoresis (SPEP). (a) Normal control band and (b) patient’s serum showing a sharp band in the mid gamma region.
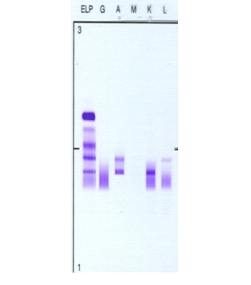
Figure 2: Patient’s serum protein immunofixation electrophoresis. Serum protein immunofixation shows two discrete IgA bands: an IgA lambda band in the beta region and an IgA kappa band in the mid-gamma region i.e. biclonal gammopathy. Key: G: IgG, A: IgA, M: IgM, K: kappa light chain, L: lambda light chain.
A 66-year-old man was seen in July 2003 for evaluation of generalized lymphadenopathy. He was asymptomatic and had no organomegaly. Blood tests showed lymphocytosis of 35×109/L. Other blood counts were normal. Serum lactate dehydrogenase (LDH) and uric acid were also normal. Direct anti-globulin test (DAT) was negative. The majority of lymphocytes appeared mature on blood film examination. Prolymphocytes were less than 5%. Bone marrow aspirate (BMA) and biopsy showed infiltration with small-sized lymphoid cells that were kappa light chain restricted, CD5/CD19/CD20/CD23 positive and CD10/CD38/FMC7 negative. Cytogenetic studies were not performed. Chest X-ray and abdomen ultrasound were normal.
The patient was diagnosed with Rai stage I CLL and received no treatment. In 2005, he developed intermittent fever, hepatosplenomegaly, increasing lymphocytosis (lymphocyte count 60×109/L), and neutropenia (absolute neutrophil count (ANC) 0.7×109/L) with rising LDH, β2 microglobulin and uric acid. His prolymphocyte count remained less than 5% and there was no evidence of large cell transformation. Serum protein electrophoresis (SPEP) showed a mild decrease in immunoglobin (Ig) M (0.32g/L; reference range 0.46–3.04) with no monoclonal band. He was treated with fludarabine and cyclophosphamide (FC). His counts normalized and he had complete regression of the lymphadenopathy and hepatosplenomegaly. However, after the fourth cycle he developed prolonged cytopenia and treatment was withheld.
He remained well until 2008, when he started to have increasing lymphadenopathy and lymphocytosis. At this time, SPEP and immunofixation showed two monocloncal bands: an IgA lambda (in the beta region) and an IgA kappa (in the gamma region) with a total IgA of 5.12g/L, IgM of 0.18g/dL and IgG of 9.6g/L [Figures 1 and 2]. The IgA kappa band in the mid gamma region was quantified as 5.2g/L. The IgA lambda band could not be quantified as it was buried in the beta region with other non-monoclonal proteins.
He was treated with chlorambucil and remained stable until February 2010 when he developed a chest infection and was found to have a lymphocyte count of 236×109/L, ANC of 0.9×109/L, hemoglobin 6g/dL (DAT negative), and platelets of 71×109/L. He had generalized lymphadenopathy and splenomegaly. BMA with repeat flowcytometry showed CD5/CD19/CD20/CD22/CD23/FMC7 positive, kappa light chain restricted lymphoid cells. Zapp 70 and CD38 were both negative. Cyclin D1 was negative and cytogenetic studies were unremarkable. Translocation of t(11;14) was not demonstrated.
He received fludarabine, cyclophosphamide and rituximab (FCR), which was complicated by febrile neutropenia and hepatitis B reactivation (which was successfully treated with entecavir). He then developed progressive lymphocytosis with evidence of bone marrow failure and enlarging lymph nodes. He received alemtuzumab but developed repeated infections and finally succumbed to multi-drug resistant acinetobacter sepsis with multiple organ failure in the setting of active disease. He did not have any other clinical, radiological, or laboratory findings to suggest concomitant multiple myeloma or other lymphoproliferative disorders.
Discussion
Besides hypogammaglobulinemia2 and the poly-reactive autoantibodies,3 patients with CLL have been shown to have monoclonal proteins. These can even precede the diagnosis of CLL.4 It is estimated that between 60% to 80% have a demonstrable monoclonal proteins in their serum.1,5 In addition, over 60% of patients have monoclonal proteins in their urine (Bence-Jones proteinuria).6 IgG kappa is the most common followed in order of frequency by IgG lambda, IgM kappa, IgM lambda, and IgA lambda.7 However, with the use of serum free light chain (FLC) assays, monoclonal FLC appear to be the most commonly detected paraproteins in CLL.8
Compared to monoclonal gammopathies, reports of biclonal proteins are very few and the finding of biclonal gammopathy in CLL is very rare. Biclonal gammopathies have been reported in multiple myeloma and in patients with gammopathies of undetermined significance.9 However, there are very few case reports of true biclonal gammopathies in CLL.10,11 In three reported cases, the combinations of monoclonal proteins included: IgG kappa and IgM kappa in one patient, and IgM kappa and IgM lambda in two patients. One patient with lambda FLC and IgG lambda simulating a biclonal gammopathy was reported.12 In addition, one case report of CLL with intracytopalsmic IgM lambda and IgG lambda inclusion.13 One rare case of CLL producing four monoclonal proteins has also been reported with the combination of IgG lambda, IgG kappa, IgA kappa, and IgM kappa.14
The impact of finding monoclonal or biclonal proteins on patients’ outcome is not very clear in CLL. In the case of monoclonal proteins, some reports suggest that there is a negative impact on patients’ survival independent of the clinical stage.7,15 Using FLC assays, it has also been shown that monoclonal FLC were associated with poor overall survival.8 For biclonal gammopathy, the clinical significance and prognostic impact in patients with CLL is uncertain. It is possible that the appearance of a second protein could be a marker of disease progression with possible transformation into a more aggressive disease, as was reported in some patients with biclonal gammopthay. Since very few cases of CLL with biclonal gammopathy have been reported, it is difficult to draw any conclusions.
Conclusion
Biclonal gammopathy is rare. Its clinical significance is, as yet, unclear given the paucity of reports. Given the rarity of such a finding, more collaborative efforts are required to accumulate enough data for more meaningful analysis and conclusions. Such results could help us to better understand the pathophysiology behind diseases like CLL and may result in better clinical prognostic systems and establish more effective and individualized therapies.
Disclosure
The authors reported no conflict of interest.
Acknowledgements
We would like to acknowledge the effort of all of our colleagues who contributed to the management of this patient. More importantly, we would like to acknowledge the patient’s family for entrusting us with his treatment.
references
- Deegan MJ, Abraham JP, Sawdyk M, Van Slyck EJ. High incidence of monoclonal proteins in the serum and urine of chronic lymphocytic leukemia patients. Blood 1984 Dec;64(6):1207-1211.
- Davey FR, Kurec AS, Tomar RH, Smith JR. Serum immunoglobulins and lymphocyte subsets in chronic lymphocytic leukemia. Am J Clin Pathol 1987 Jan;87(1):60-65.
- Hamblin TJ. Autoimmune complications of chronic lymphocytic leukemia. Semin Oncol 2006 Apr;33(2):230-239.
- Tsai HT, Caporaso NE, Kyle RA, Katzmann JA, Dispenzieri A, Hayes RB, et al. Evidence of serum immunoglobulin abnormalities up to 9.8 years before diagnosis of chronic lymphocytic leukemia: a prospective study. Blood 2009 Dec;114(24):4928-4932.
- Beaume A, Brizard A, Dreyfus B, Preud’homme JL. High incidence of serum monoclonal Igs detected by a sensitive immunoblotting technique in B-cell chronic lymphocytic leukemia. Blood 1994 Aug;84(4):1216-1219.
- Pezzoli A, Pascali E. Monoclonal Bence Jones proteinuria in chronic lymphocytic leukaemia. Scand J Haematol 1986 Jan;36(1):18-24.
- Noel P, Kyle RA. Monoclonal proteins in chronic lymphocytic leukemia. Am J Clin Pathol 1987 Mar;87(3):385-388.
- Maurer MJ, Cerhan JR, Katzmann JA, Link BK, Allmer C, Zent CS, et al. Monoclonal and polyclonal serum free light chains and clinical outcome in chronic lymphocytic leukemia. Blood 2011 Sep;118(10):2821-2826.
- Kyle RA, Robinson RA, Katzmann JA. The clinical aspects of biclonal gammopathies. Review of 57 cases. Am J Med 1981 Dec;71(6):999-1008.
- Schipper H, Orr KB, Bow EJ. Coexistence of double gammopathy (IgM kappa and IgM lambda) in the serum of a single individual with chronic lymphocytic leukemia. Acta Haematol 1983;69(1):23-31.
- Schaffner KF, Krause JR, Kelly RH. Biclonal IgM gammopathy in chronic lymphocytic leukemia. Arch Pathol Lab Med 1988 Feb;112(2):206-208.
- Guastafierro S, Celentano M, Cuomo C, Falcone U. Chronic lymphocytic leukemia with associated lambda-light-chain and IgG lambda paraproteins simulating a biclonal gammopathy. Clin Lab 2010;56(11-12):577-580.
- White V, Boyko WJ. Chronic lymphocytic leukemia with IgM lambda and IgG lambda cytoplasmic inclusions. Arch Pathol Lab Med 1983 Nov;107(11):580-582.
- Tienhaara A, Irjala K, Rajamäki A, Pulkki K. Four monoclonal immunoglobulins in a patient with chronic lymphocytic leukemia. Clin Chem 1986 Apr;32(4):703-705.
- Bernstein ZP, Fitzpatrick JE, O’Donnell A, Han T, Foon KA, Bhargava A. Clinical significance of monoclonal proteins in chronic lymphocytic leukemia. Leukemia 1992 Dec;6(12):1243-1245.