Type 2 diabetes mellitus (T2D) results in the progression of hyperglycemia with time, and can cause multiple organ damage. Long-term complications of hyperglycemia include heart disease, stroke, diabetic retinopathy, kidney failure, and poor circulation of limbs leading to amputations. Accordingly, glycemic control and associated conditions need to be assessed and monitored frequently. Glycosylated hemoglobin (HbA1C) provides the main method by which clinicians can relate individual’s glycemic control to risk of complication development.1,2
Many people with diabetes have an elevated blood pressure. High blood pressure is associated with a spectrum of adverse outcomes, including eye damage, kidney damage, cardiovascular disease (CVD), and premature mortality. Treatment to reduce elevated blood pressure reduces these adverse outcomes.1,2
CVD is the major cause of morbidity and mortality in people with T2D. Assessment and management of CVD risk factors in T2D is a core part of diabetic care. Dyslipidemia is a well-recognized and modifiable risk factor that should be identified early to institute aggressive cardiovascular preventive management.3 Evaluation is achieved by the measurements of serum lipid levels, especially low-density lipoprotein (LDL), cholesterol, and triglycerides. The most common form of diabetic dyslipidemia consists of moderate elevation in triglyceride levels, low high-density lipoprotein (HDL) cholesterol, and high level of LDL cholesterol. However, LDL cholesterol levels in patients with T2D are generally similar to those found in the general population.3 In the UK Prospective Diabetes Study (UKPDS) triglyceride levels did not predict coronary heart disease (CHD) events. High LDL cholesterol was the strongest independent predictor of CHD followed by low HDL cholesterol levels.4 This supports current guidelines in which lowering LDL cholesterol is the primary lipid target.
All patients with diabetes, even those with normal lipid profiles, will likely benefit from statin use. There is strong evidence that statins reduce the risk of CVD or death events irrespective of age and gender, across a wide range of cholesterol levels.5-7 In addition, for people with established CVD, the benefit of long-term aspirin use for reducing the risk of myocardial infarction (MI), stroke, and vascular death is well established.8,9
Diabetes is now the leading cause of chronic kidney disease (CKD) in many developed countries. The prevalence of CKD in people with T2D varies between 25–50%.1 The two main manifestations of CKD in people with T2D are a reduction in glomerular filtration rate, or the presence of albuminuria/proteinuria.1 Treatment in the early stages of CKD reduces progression of kidney damage. Therefore, patients with T2D should be screened regularly (at diagnosis and then annually) to detect early indications of kidney damage and receive treatment.
In Oman, most epidemiological studies on T2D were done in primary health care centers where new, controlled, and less advanced cases of T2D patients were seen.10-12 This study assessed the quality of diabetes care at Sultan Qaboos University Hospital (SQUH), a countrywide tertiary referral center in Oman, through the evaluation of HbA1C, blood pressure, serum lipids and urine albumin/creatinine ratio in a sample of Omani T2D patients. The assessment was done using the American Diabetes Association (ADA) standards of medical care in diabetes and guidelines from the American National Cholesterol Education Program III NCDP NIII Adult Treatment Panel (ATP).
Methods
SQUH is one of the two main referral hospitals in Oman, catering for a catchment area including more than 2,000,000 people. In 2010, 3,003 people of different ages and nationalities with diabetes were registered at SQUH. About half of those were Omanis (n=1687). Of those, 673 adults with T2D were included in the study.
We performed a retrospective, observational, cross-sectional study using Omani T2D patients that were seen at the diabetes (n= 523) and family medicine (n= 150) clinics at SQUH. We collected patient data from June 2010 to February 2012 from the Hospital Information System (HIS). A history of T2D among patients was determined using diagnosis and medical history stored in the electronic records of the HIS. Patients had to be Omani, aged more than 18 years old, and have T2D with active follow-up and at least three visits within one year to either clinic to be included in the study. Exclusion criteria included: patients with type 1 diabetes, positive antibodies testing for islet cell antibodies and glutamic acid decarboxylase antibodies, or patients diagnosed with cancer. Participants were informed about the project and written consent obtained. The study was approved by the Ethics and Research Committee of the College of Medicine, Sultan Qaboos University, Muscat, Oman.
Anthropometric variables collected included: age, gender, height, and weight [Table 1]. Obesity status was defined according to the international classification of an adult’s weight, their body mass index (normal BMI: 18.5–24.99kg/m2, overweight: 25.00–29.99kg/m2 and obese ≥30.00kg/m2). The biochemical investigations retrieved were: HbA1C level, serum lipids (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides) and urine microalbumin/creatinine ratio (ACR) [Table 1]. Blood pressure and duration of diabetes
were also noted.
In this study for assessment purposes, the American Diabetes Association (ADA) standards for medical care in diabetes (2013) were used.2,13 Glycemic control was defined by the HbA1C goal of <7%. HbA1C control was divided into three categories: optimal (<7%), fair (7.0–8.9%), and poor control3 (9%). Blood pressure goal was defined as systolic <140mmHg and diastolic <80mmHg.13 The guidelines of American National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III (2001/2004) was used for serum lipids goals.14,15 Total cholesterol (desirable <5.17mmol/L); LDL cholesterol (optimal <2.59mmol/L); triglycerides (desirable <1.69mmol/L); and HDL cholesterol (high >1.55mmol/L). In addition, urinary ACR values were divided into four categories: normal values (≤2.5mg/mmol (men), ≤3.5mg/mmol (women)); microalbuminuria (2.6–29mg/mmol (men), 3.6–29mg/mmol (women)); clinical proteinuria (30–60mg/mmol); overt nephropathy/heavy proteinuria (≥70 mg/mmol).1,2
Data analyses were performed using SPSS (version 20.0). Anthropometric data was expressed as mean ±SD and percentage. Age was divided into four intervals (≤35, 36–50, 51–65, and >65 years), and the percentage of patients in each age group was calculated. Duration of diabetes was also divided into five intervals (≤5, 6–10, 11–15, 16–20, and >20 years). The percentage of patients in each interval was calculated. In addition, the percentage of patients who reached the recommended goals for HbA1C, blood pressure, serum lipids, and urine ACR was calculated and used as indicator outcomes to assess the care given to patients with T2D.
Results
Table 1: Characteristics of Omani patients (n=673) with T2D seen at Sultan Qaboos University Hospital, Muscat, Oman.
|
Gender (n=669) |
|
Male |
310 (46) |
|
|
Female |
359 (54) |
|
Age (years), (n=670) |
|
≤35 |
21 (3) |
54 (±10) |
|
>35–50 |
202 (30) |
|
>50–65 |
357 (53) |
|
>65 |
90 (13) |
|
BMI (n=541) |
|
<18.5 (underweight) |
1 (0.2) |
31 (±6) |
|
18.5–24.99 (normal weight) |
57 (11) |
|
25.00–29.99 (overweight) |
189 (35) |
|
≥ 30.00 (obese) |
294 (54) |
|
Duration of diabetes (years), (n=562) |
|
≤5 |
174 (31) |
11 (±8) |
|
>5–10 |
155 (28) |
|
>10–15 |
90 (16) |
|
>15–20 |
102 (18) |
BMI: Body mass index, SD: Standard deviation.
A total of 673 Omani patients with T2D were included in our study. The mean ±SD age of patients was 54 ±10 years, ranging from 30 to 84 years. Over half of patients were in the 51–65 years age group. Forty six percent were males and 54% females. The mean duration of diabetes was 11 ±8 years, ranging from less than 1 year to 40 years. Almost three-quarters (69%) had diabetes for more than
five years [Table 1].
Almost all patients (n=622, 93%) were on treatment, either oral hypoglycemic drugs and/or insulin. Thirty-nine patients (6%) had no record of the type of drugs used, and 10 patients (1%) did not use any drugs. During the observation period insulin was prescribed to 278 patients (41%). Only 27 patients (4%) documented refusing insulin treatment. Aspirin was prescribed to 423 patients (63%), while statins (simvastatin and rosuvastatin) were prescribed to 470 patients (70%).
Twenty-two percent of patients had HbA1C less than 7%, and 47% had blood pressure less than <140/80mmHg. For serum lipids, 48% and 67% of patients achieved the LDL cholesterol goal of less than 2.6mmol/L, and triglyceride goal of less than1.7mmol/L, respectively. While, 59% of males and 43% of females achieved the HDL cholesterol goals (males >1.0mmol/L, females >1.3mmol/L). For urinary ACR, 60% of patients were within normal range [Table 2 and Figure 1].
On the other hand, 41% of the patients had poor HbA1C control (≥9%). Forty-six percent had systolic blood pressure ≥140mmHg and 39% had diastolic blood pressure ≥80mmHg. Twenty six percent of patients had microalbuminuria, while 14% of patients had either clinical proteinuria or
overt nephropathy.
Table 2: Characteristics of clinical variables of Omani patients with T2D (n=673) seen at Sultan Qaboos University Hospital, Muscat, Oman.
|
HbA1C (%), (n=673) |
|
|
|
Optimal < 7% (< 53mmol/mol) |
150 (22) |
8.7 ± 2.1 %
(72 mmol/mol) |
|
Fair ≥7 to < 9 % (≥ 53 to 75 mmol/mol) |
245 (36) |
|
Poor ≥ 9 % (≥ 75 mmol/mol) |
277 (41) |
|
Hypertension optimal control, (n=673) |
|
<140/80mmHg |
319 (47) |
|
|
Systolic blood pressure, (n=673) |
|
≤120mmHg |
110 (16) |
140 ± 20 |
|
>120–139mmHg |
255 (38) |
|
140–159mmHg |
200 (30) |
|
≥160 mmHg |
108 (16) |
|
Diastolic blood pressure, (n=673) |
|
<80mmHg |
412 (61) |
77 ± 11 |
|
80–89mmHg |
187 (28) |
|
90–99mmHg |
63 (9) |
|
>100mmHg |
10 (2) |
|
Lipid targets |
|
Total cholesterol (n= 657) |
|
Desirable: <5.17mmol/L (<200mg/dL) |
474 (72.1) |
4.7 ± 1.1 |
|
Borderline high: 5.17–6.18mmol/L (200–239mg/dL) |
130 (19.8) |
|
High: ≥6.2mmol/L (≥240mg/dL) |
53 (8.1) |
|
LDL cholesterol (n=644) |
|
Optimal: <2.59mmol/L (<100mg/dL) |
307 (47.7) |
2.7 ± 0.96 |
|
Near optimal/above optimal: 2.59–3.33 mmol/L (100–129mg/dL) |
193 (30) |
|
Borderline high: 3.36–4.1 mmol/L (130–159 mg/dL) |
91 (14.1) |
|
High: 4.14–4.89 (160–189mg/dL) |
34 (5.3) |
|
Very high: >4.91mmol/L (≥190mg/dL) |
19 (2.9) |
|
Triglyceride (n=655) |
|
Desirable: <1.69mmol/L (<150mg/dL) |
440 (67.2) |
1.6 ± 0.95 |
|
Borderline high: 1.69–2.25mmol/L (150–199mg/dL) |
114 (17.4) |
|
High: 2.26–5.63 mmol/L (200–499mg/dL) |
97 (14.8) |
|
Very high: >5.65mmol/L (≥500mg/dL) |
4 (0.61) |
|
HDL cholesterol (n=656) |
|
Low: <1mmol/L (<40mg/dL) |
200 (30.5) |
1.2 ± 0.47 |
|
Intermediate: 1–1.55mmol/L (40–60mg/L) |
363 (55.3) |
|
High (Protective against heart disease):
>1.55mmol/L (≥60mg/dL) |
93 (14.2) |
|
Urinary microalbumin/creatinine ratio (n= 655) |
|
Normal: ≤2.5mg/mmol (males), ≤3.5mg/mmol (females) |
391 (60) |
|
|
Microalbuminuria: 2.6–29mg/mmol (males), 3.6–29mg/mmol (females) |
173 (26) |
|
Clinical proteinuria: 30–60mg/mmol |
30 (5) |
SD: Standard deviation; LDL: Low density lipoprotein; HDL: High density lipoprotein; HBA1c: Glycosylated hemoglobin
Discussion
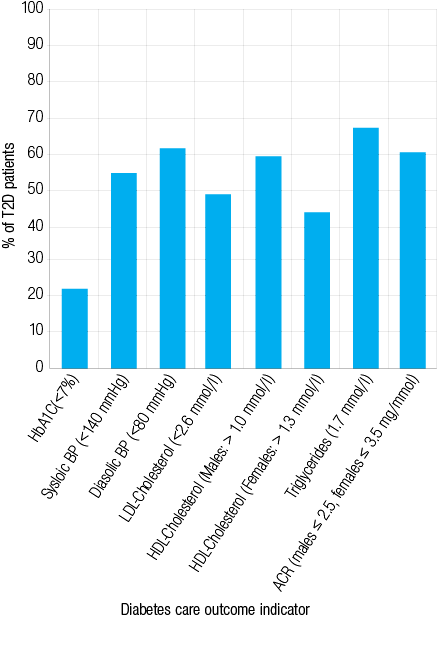
Figure 1: Proportion of patients with type 2 diabetes mellitus patients reaching the American Diabetes Association standards of medical care in diabetes at Sultan Qaboos University Hospital, Muscat, Oman.
This study describes and assesses the clinical care given to a sample of Omani patients with advanced and uncontrolled cases of T2D attending SQUH, a tertiary hospital. Using the ADA standards of medical care in diabetes,2,13 22% of the patients achieved HbA1C goal, 47% achieved the blood pressure goal, 48% achieved serum LDL cholesterol goal, 67% achieved serum triglycerides goal, and 59% of males and 43% of females achieved serum HDL cholesterol goal. In addition, almost 60% of the patients had urinary ACR within the normal range. The patients included in this study were middle aged and elderly with a mean age of 54 ±10 years, and 69% had diabetes for more than five years. Additionally, 35% were overweight and 54% were obese.
Omani patients get free medical care including free medication at government hospitals. Most patients with T2D are seen, on average, four times annually at primary health care centers. Advanced and uncontrolled cases of patients with T2D are referred to SQUH, expecting optimum control of those patients. In this study, the proportion of patients who reached HbA1C goal was lower than those obtained from a previous study conducted at SQUH.16 Other Omani studies, done at primary health care centers, also reported a higher number of patients (24–30%) with glycemic control.10,12
The clinical outcomes from care that patients with T2D received at SQUH did not match those from Europe,17,18 North America,19 Canada,20 and Australia.21 Compared to reports from similar tertiary centers in Europe,17 Canada,20 and Australia,21 clinical outcomes in Omani patients were lower. For example, 42% of the patients in Portugal, USA, and Canada were at HbA1C goal (<7.0%)17,19 compared to 22% in Oman.
Although we rated the quality of T2D management among Omani patients as inadequate, the outcomes are similar, and in some cases better than those reported from other tertiary diabetes centers in the Arab region.22,23 For example, only 21–28% of the patients in the region met the HbA1C level goal. However, a recent study in a tertiary hospital in the United Arab Emirates24 showed an improvement in diabetes care outcomes, from 2008 to 2010, comparable to developed countries.
Hypertension is a disease that is associated with diabetes, and is known to contribute to diabetic microvascular and macrovascular complications. In this study, 47% of patients with T2D had their blood pressure at goal. Previous studies among Omani patients, at primary health care centers, reported similar results.10-12 Our blood pressure control results are similar to tertiary centers in Saudi Arabia, the United Arab Emirates, and Lebanon.22-24 Other studies reported it at 11–36%.25,26 However, different criteria for blood pressure control among diabetics was used in different studies, which makes evaluation difficult.
A specific dyslipidemia phenotype is particularly common in patients with diabetes.27-30 In our study, 48% were at the goal for LDL cholesterol. In previous studies at primary care centers in Oman, only 15–24% of the patients achieved LDL cholesterol goal.10,12 Our study showed better management of lipids among patients attending SQUH. Approximately, two-thirds of the patients with diabetes attending SQUH were on aspirin and statins treatment to reduce the risk of CVD events or death. However, lipid management outcomes in patients attending SQUH were lower than those from tertiary centers in the Arabian Gulf.22,24
Microalbuminuria may be used as an early indicator of diabetic nephropathy. In this study, 26% of patients had microalbuminuria. A previous study among Omani patients at SQUH showed similar findings.31 However, Al-Lawatiya et al,12 reported a higher figure (37%) in patients attending primary health care centers. Prashanth et al,32 estimated the prevalence of microalbuminuria in patients with T2D among three Arabian Gulf countries (Bahrain, UAE and Oman) at 28–35%. However, high rates of microalbuminuria were reported among patients attending different levels of health care in Saudi Arabia (41%) and India (36%).33,34 Epidemiological studies reported the prevalence of microalbuminuria, approximately 10 years after the diagnosis of T2D, between 25–40%.35-38
Despite the impression from this study that Omani patients with T2D are not well controlled at SQUH, baring in mind the patient’s older age, long duration of diabetes, advanced cases, and the late presentation of the disease SQUH receives, the clinical outcome attained for care given to patients with T2D could be considered acceptable. In addition, many barriers impact on achievement of the recommended care such as the reluctance of Omani patients to accept insulin therapy, which delays the proper treatment in complicated cases. Another is low compliance with treatment plans. A third problem is the traditional dietary habits (traditional Omani food is high in carbohydrates, fats and sugars) combined with the increasing prevalence of sedentary lifestyles and reduced physical activity. Therefore, improving the outcome of the clinical care given to Omani T2D patients, such as those found in this study, is a challenge for health care providers who need to address multifactorial issues in their management plans.
Conclusion
The goals of clinical outcome attained for care given to patients with T2D at SQUH were lower than those reported in Europe, North America, Canada, and Australia. However, it is similar, and sometimes better, than those reported in the Arab region. There is scope for better control and a higher number of patients reaching recommended targets, but many barriers in the Omani community impact on the recommended care, particularly educational and lifestyle factors.
Diabetes is a complex disease; treatment requires multiple processes involving both provider and patient. In our community, that should also include patient education, the importance of lifestyle modification, altering the traditional dietary habits, increasing physical activity and training of primary care physicians.
Disclosure
The authors declared no conflict of interests. This project was supported by the Research Council (TRC), Muscat, Oman (grant number RC/MED/BIOC/10/01).
Acknowledgment
We thank Nassra Al Maani and Ranjitha K. Sukumaran for their contribution in data collection. We also thank George Khaukha and Taruna Dutt for their support. We are grateful to the staff of the Diabetes and FAMCO clinics at SQUH for their help and support. We are indebted to all subjects who participated in this study. We are grateful to the Deanship of postgraduate studies at Sultan Qaboos University, Muscat, Oman for the PhD grant to Sawsan Al-Sinani.
references
- IDF. Global Guideline for Type 2 Diabetes. International Diabetes Federation; 2012 [cited 2012]; Available from http://www.idf.org/global-guideline-type-2-diabetes-2012.
- ADA. Standards of medical care in diabetes 2013. Diabetes Care 2013;36(1):S11-S66.
- Solano M, Goldberg R. Lipid management in type 2 diabetes. Clin Diabetes 2006;24(1):27-32 .
- Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998 Mar;316(7134):823-828.
- Brugts J, Yetgin T, Hoeks S, Gotto A, Shepherd J, Westendorp R, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009;338.
- Taylor F, Ward K, Moore T, Burke M, Davey Smith G, Casas J, et al. Statins for the primary prevention of cardiovascular disease. Cochrane database of systematic reviews. 2011;(1):CD004816.
- Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol 2009 May;8(5):453-463.
- Baigent C, Sudlow C, Collins R, Peto R; Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002 Jan;324(7329):71-86.
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al; Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009 May;373(9678):1849-1860.
- Al-Mandhari A, Al-Zakwani I, El-Shafie O, Al-Shafaee M, Woodhouse N. Quality of Diabetes Care: A cross-sectional observational study in Oman. Sultan Qaboos Univ Med J 2009 Apr;9(1):32-36.
- El-Shafie K, Rizvi S. Control of Hypertension among Type II Diabetics. Oman Med J 2010 Jan;25(1):32-36.
- Al-Lawati JA, N Barakat M, Al-Zakwani I, Elsayed MK, Al-Maskari M, M Al-Lawati N, et al. Control of risk factors for cardiovascular disease among adults with previously diagnosed type 2 diabetes mellitus: a descriptive study from a middle eastern arab population. Open Cardiovasc Med J 2012;6(1):133-140.
- ADA. Executive summary: Standards of medical care in diabetes--2013. Diabetes Care 2013;36(1):S4-S10.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001 May;285(19):2486-2497.
- CDC. State Heart Disease and Stroke Prevention Programs Address High Blood Cholesterol. 2007 [cited 2014 December]; Available from: http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_state_cholesterol.htm.
- Al-Maskari MY, Al-Shookri AO, Al-Adawi SH, Lin KG. Assessment of quality of life in patients with type 2 diabetes mellitus in Oman. Saudi Med J 2011 Dec;32(12):1285-1290.
- Panarotto D, Teles AR, Schumacher MV. Factors associated to glycaemic control in patients with type 2 diabetes. Rev Assoc Med Bras 2008 Jul-Aug;54(4):314-321.
- Khunti K, Gadsby R, Millett C, Majeed A, Davies M. Quality of diabetes care in the UK: comparison of published quality-of-care reports with results of the Quality and Outcomes Framework for Diabetes. Diabet Med 2007 Dec;24(12):1436-1441.
- Saaddine JB, Cadwell B, Gregg EW, Engelgau MM, Vinicor F, Imperatore G, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988-2002. Ann Intern Med 2006 Apr;144(7):465-474.
- Malcolm JC, Maranger J, Taljaard M, Shah B, Tailor C, Liddy C, et al. Into the abyss: diabetes process of care indicators and outcomes of defaulters from a Canadian tertiary care multidisciplinary diabetes clinic. BMC Health Serv Res 2013;13(1):303.
- Bryant W, Greenfield JR, Chisholm DJ, Campbell LV. Diabetes guidelines: easier to preach than to practise? Med J Aust 2006 Sep;185(6):305-309.
- Kharal M, Al-Hajjaj A, Al-Ammri M, Al-Mardawi G, Tamim HM, Salih SB, et al. Meeting the American Diabetic Association standards of diabetic care. Saudi J Kidney Dis Transpl 2010 Jul;21(4):678-685.
- Akel M, Hamadeh G. Quality of diabetes care in a university health center in Lebanon. Int J Qual Health Care 1999 Dec;11(6):517-521.
- Alhyas L, Cai Y, Majeed A. Type 2 diabetes care for patients in a tertiary care setting in UAE: a retrospective cohort study. JRSM Short Rep 2012 Oct;3(10):67.
- Pérez-Cuevas R, Doubova SV, Suarez-Ortega M, Law M, Pande AH, Escobedo J, et al. Evaluating quality of care for patients with type 2 diabetes using electronic health record information in Mexico. BMC Med Inform Decis Mak 2012;12:50.
- Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004 Jan;291(3):335-342.
- Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia 2003 Jun;46(6):733-749.
- Krauss RM, Siri PW. Dyslipidemia in type 2 diabetes. Med Clin North Am 2004 Jul;88(4):897-909, x.
- Del Pilar Solano M, Goldberg RB. Management of diabetic dyslipidemia. Endocrinol Metab Clin North Am 2005 Mar;34(1):1-25, v.
- Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 2009 Mar;5(3):150-159.
- Al-Futaisi A, Al-Zakwani I, Almahrezi A, Al-Hajri R, Al-Hashmi L, Al-Muniri A, et al. Prevalence and predictors of microalbuminuria in patients with type 2 diabetes mellitus: a cross-sectional observational study in Oman. Diabetes Res Clin Pract 2006 May;72(2):212-215.
- Prashanth P, Sulaiman KJ, Kadaha G, Bazarjani N, Bakir S, El Jabri K, et al; DEMAND Gulf Study Investigators. Prevalence and risk factors for albuminuria among type 2 diabetes mellitus patients: a Middle-East perspective. Diabetes Res Clin Pract 2010 Jun;88(3):e24-e27.
- Al-Khader AA. Impact of diabetes in renal diseases in Saudi Arabia. Nephrol Dial Transplant 2001 Nov;16(11):2132-2135.
- Varghese A, Deepa R, Rema M, Mohan V. Prevalence of microalbuminuria in type 2 diabetes mellitus at a diabetes centre in southern India. Postgrad Med J 2001 Jun;77(908):399-402.
- Newman D, Mattock M, Dawnay A, Kerry S, McGuire A, Yaqoob M, et al. Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess. 2005;9(30):iii-vi, xiii-163.
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008 Jun;358(24):2560-2572.
- Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003 Jan;63(1):225-232.
- Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG; DEMAND investigators. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006 Jun;69(11):2057-2063.