Ultrasound Location of Misplaced Levonorgestrel Releasing
Intrauterine System (LNG-IUS) – is it easy?
Vaidyanathan Gowri, Mariam Mathew
ABSTRACT
The Levonorgestrel intrauterine device (LNG-IUD) is a hormone-containing device licensed for treatment of menorrhagia and contraception. Though complications such as perforation have been reported similar to other non-hormonal intrauterine devices, the diagnosis of such complications is difficult with this device because the LNG-IUD has a different ultrasound appearance compared to copper devices and these case reports are intended to emphasize this point.
From the Department of Obstetrics and Gynecology, Sultan Qaboos University Hospital.
Received: 12 Aug 2008
Accepted: 04 Nov 2008
Address correspondence and reprint requests to: Dr. Vaidyanathan Gowri, Assistant Professor, Department of Obstetrics and Gynecology, Sultan Qaboos University Hospital, PO Box 35; Postal Code 123, Muscat, Oman.
E-mail: gowrie61@hotmail.com
.
The levonorgestrel intrauterine system (LNG–IUS Mirena, Schering AG, Germany) is a small, T-shaped intrauterine system. Complaints of lower abdominal pain following IUS insertion should always be investigated. Ultrasound location of the LNG-IUS is not as easy as for copper‑containing intrauterine devices as it is not uniformly hyperechogenic. The LNG-IUS is hormonally active in spite of perforation. These case reports are intended to emphasize these two points. Laparoscopic retrieval is possible, in the event of a uterine perforation into the peritoneal cavity.
Mrs. A, para 4 had an LNG-IUS inserted three months after her delivery. She was lactating and amenorrheic at the time of LNG-IUS insertion. Two days after insertion of the intrauterine device, she went to a nearby hospital for pain in the abdomen. An x-ray of the abdomen was done and the intrauterine device was thought to be outside the uterus.
Abdominal examination was unremarkable and on vaginal speculum examination the threads were not seen. Bimanual examination revealed a normal sized uterus and no adnexal tenderness. A transvaginal ultrasound scan was inconclusive (intrauterine device seen near the fundus of the uterus). She underwent hysteroscopy/laparoscopy the same week.Hysteroscopy was normal and on laparoscopy the LNG-IUS was found in the pouch of Douglas lying freely with no adhesions. The uterus, tubes and ovaries were normal. The device was removed easily.
Mrs. Z, a para 1, presented to the gynecology clinic three months after insertion of an LNG-IUS with history of heavy vaginal bleeding of 10 days’ duration. She gave history of irregular vaginal bleeding since the insertion of the device, which was done approximately three months earlier. Her last delivery was five months ago and Mirena was inserted six weeks post-delivery. She went to a nearby clinic for removal of the device, where a transvaginal scan was done and she was reassured that the device was in the uterus. Abdomen was soft and there was no tenderness. In our hospital, on speculum examination, the threads were not visible. Bimanual examination was normal. A transvaginal ultrasound in our hospital did not show the device in the uterus. Plain x-ray of the abdomen revealed the device in the pelvis, probably outside the uterus. She underwent hysteroscopy and laparoscopy. Hysteroscopy was normal and the laparoscopy revealed that the LNG‑IUS was in the pouch of Douglas with minimal adhesions to the fimbrial end of the fallopian tube. The adhesions were released and the device was removed easily.
Mrs. J., a para 3, presented two years after insertion of Mirena with history of lower abdominal pain but no fever or vomiting. Her last child is 2 years and 3 months of age. She was amenorrheic after the insertion of Mirena. According to her history, she was fine after the insertion of the LNG-IUS and follow-up visits showed the threads of the device on speculum examination. Her last follow-up was eight months prior to admission. On examination, the abdomen was soft and non-tender with no palpable masses. On vaginal speculum examination, the threads were not visible and bimanual examination was normal. Transvaginal ultrasound was inconclusive and a Computed Tomography (CT) scan was performed. The CT scan showed the device outside the uterus. She consented for laparoscopy and laparotomy. On laparoscopy, the Mirena was found in the upper abdomen embedded in omental adhesions. The adhesions were released and the device was removed easily.
The incidence of intrauterine device perforation is 0.9% and this includes perforation into other abdominal organs.1 Houdenhovena et al reported the incidence of uterine perforations related to the insertion of a LNG-IUS as 2.6 per 1,000 insertions. Insertion in lactating women even beyond six weeks after delivery is shown to be an important risk factor in their study.2 All three patients in this report were lactating at the time of LNG-IUS insertion. Usually, the first investigation when a woman presents with lost intrauterine device is ultrasound of the pelvis, preferably transvaginal. LNG-IUS has a typical sonographic appearance, differing from that of regular IUDs.3 Its sonographic appearance includes both proximal and distal ends of the vertical arm of the device, which extend into the internal cervical os and fundal region, respectively. Acoustic shadowing between both ends defines the location of the device, which should help avoid consultations due to “lost IUDs.” We could not be sure in two of our patients whether the LNG‑IUS was in the uterus, by transvaginal scan.
Peritoneal adhesion formation has been described with LNG-IUS and is similar to that of copper-bearing IUCD.4 In the second patient, the adhesions were minimal, but the LNG-IUS was buried in omental adhesions in the third patient. It is concluded by Nitke et al that lost LNG‑IUSs are associated with a higher rate of localization errors by clinical evaluation than copper intrauterine devices.5 Lost Levonorgestrel-releasing IUDs are found in the mid-upper abdomen, embedded in omental tissue, and they suggest that the upper abdomen should be explored first during laparoscopy.
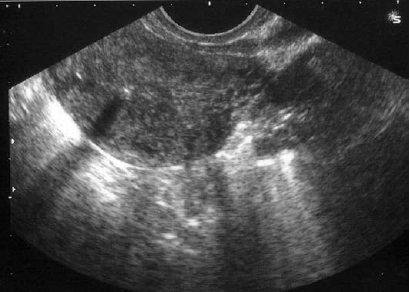
Figure 1: Ultrasound appearance of the Mirena coil
The recommended management of a misplaced LNG-IUS by the manufacturers is laparoscopic removal. In the case of conservative management, intraabdominal LNG-IUS results in very high plasma LNG levels and, therefore, must be removed if pregnancy is desired. However, in our patients, plasma levels of Levonorgestrel were not done (prior to removal or after removal) to suggest that there was hormonal activity in spite of perforation.4
-
Anderson K, Ryde-Blomqvist E, Lindell K, Odlind V, Milsom I. Perforation with intrauterine devices. Report from a Swedish survey. Contraception 1998; 57:251-255.
-
Van Houdenhoven K, van Kaam KJ, van Groothest AC, Salemans TH, Dunselman GA, Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception 2006; 73:257-260.
-
Zalel Y, Kreizer D, Soriano D, Achiron R. Sonographic demonstration of a levonorgestrel-releasing IUD (Mirena). Harefuah. 1999; 137:30-31.
-
Haimov-Kochman R, Doviner V, Amsalem H, Prus D, Adoni A, Lavy Y. Intraperitoneal Levonorgestrel-releasing intrauterine device following uterine perforation: the role of progestins in adhesion formation. Human reproduction 2003; 18:990-993.
-
Nitke S, Rabinerson D, Dekel A, Sheiner E, Kaplan B, Hackmon R. Lost levonorgestrel IUD: diagnosis and therapy. Contraception 2004; 69:289-293.