|
Abstract
Follicular dendritic cell sarcoma arises from the follicular dendritic cells present in the lymph nodes, especially of the head and neck area. Rarely, extranodal sites may be affected including tonsil, the oral cavity, liver, spleen and the gastrointestinal tract. Here, we report a case of intra-abdominal follicular dendritic cell sarcoma presenting as a huge mass in the ileocecal region of a 24-year young woman.
Keywords: Follicular dendritic cell sarcoma; Ileocecal; Extranodal.
Introduction
Follicular dendritic cell (FDC) sarcoma is an extremely rare neoplasm arising from dendritic cells of germinal centers. Approximately 30% of the cases are reported to have been located at extranodal sites, such as the liver, tonsil and intra-abdominal soft tissue.1 Involvement of the gastrointestinal tract is extremely rare. To our knowledge, only five cases have been described to date and these were mostly located in the colon and retroperitoneum.2,3 We report of a case of follicular dendritic cell sarcoma involving the ileocaecal region of the gastrointestinal tract of a 24-year-old woman.
Case Report
A 24-year-old female presented with complaints of intermittent pain in the right iliac fossa for one year and heaviness in the lower abdomen for four months. There was no history of vomiting, abdominal distension, bleeding per rectum, fever, or past history of tuberculosis. On physical examination, there was a firm, nontender lump, palpable in the right iliac fossa. Ultrasonography of the whole abdomen showed ileocolic intussusception with suspicious hypoechoic space-occupying lesion (SOL). CT scan of the whole abdomen (Fig. 1) was suspicious for ileocolic intussusception with thickened ileal wall. Proximal small bowel loops were not distended. One globular, relatively well-defined SOL (68 × 74 mm) was noted anterior to the ascending colon and cecum. The mass was heterogeneously enhancing with small irregular hypodense area, adherent with cecum. Another globular mass (68 × 99 mm) of similar attenuation with necrotic centre was noted in the lower paravertebral location extending to the right iliac fossa. Fewer much smaller but similar globular masses were also noted in the prevertebral location below the horizontal level of the kidneys. All these globular masses were adherent with each other, but no ascites were noted besides the aorta and IVC were normal. USG-guided FNAC showed poorly differentiated carcinoma. Serum CEA was 0.5 ng/mL, LDH was 579.2 U/L and other routine laboratory examination and chest X-ray were all normal. The patient then underwent right hemicolectomy with excision of the retroperitoneal mass.
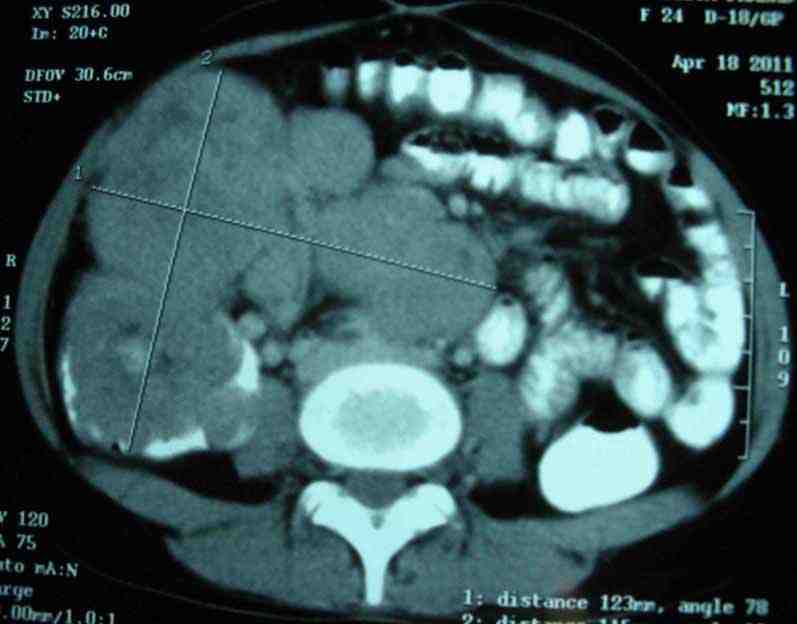
Figure 1: CT scan of whole abdomen showing one SOL adhered to ceacum and another SOL in the lower paravertebral location.
On gross examination, a polypoid tumor was seen at the ileocecal region, 6.5 cm in its greatest axis and the peri-intestinal fibro-fatty tissue showed five fleshy nodular masses, the largest measuring 11 cm in its greatest axis. Histopathological examination of both masses showed a tumor composed of interlacing fascicles of spindle-shaped cells with elongated, plump nuclei admixed with non-neoplastic small lymphocytes (Fig. 2). The tumor cells showed nuclear pleomorphism and 7-8 mitotic figures per 10 high power fields. At places, the tumor cells were arranged in a vaguely storiform pattern. The mucosa overlying the tumor in the ileocecal region was ulcerated. The histological picture was suspicious for follicular dendritic cell sarcoma. The surgical lines of resection were free of lesion.
On immunohistochemistry, the tumor cells express CD35, CD23, and CD21 (Figs. 3 and 4) and was immunonegative for CD34, CD45, CD19, c-kit, DOG-1, EMA, CK, EBV, Desmin, SMA and S-100 protein. Thus, the diagnosis of follicular dendritic cell sarcoma was confirmed. CT scan of the whole abdomen (Fig. 5) after 6 months of operation showed no abnormality in the abdomen. No adjuvant treatment was recommended for this patient. Follow-up was done with monthly clinical examination and CT scan of whole abdomen every six months for the last one year and showed no recurrence.
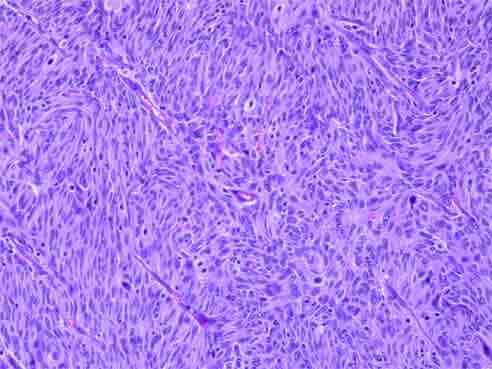
Figure 2: Histopathological examination showing interlacing fascicles of spindle shaped cells with elongated, plump nuclei admixed with non-neoplastic small lymphocytes.
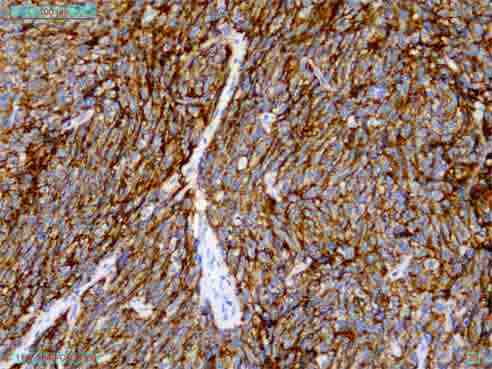
Figure 3: Immunohistochemistry showing CD 35 positive.
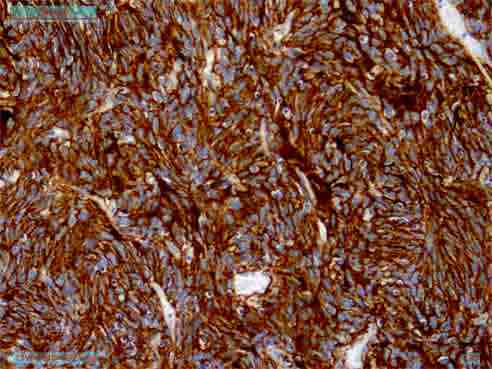
Figure 4: Immunohistochemistry showing CD 21 positive.
Figure 5: CT scan of whole abdomen after 6 months of operations showing no abnormal mass.
Discussion
FDC sarcoma is a very rare neoplasm. It was first described in 1986 by Monda et al. and there are less than 80 cases reported in the English literature.4-7 This neoplasm affects both sexes equally, and the disease occurs over a wide age range from 14 to 77 years (mean age: 47 years). The disease typically presents as a painless, slow growing, well-defined mass lesion at the involved site.8
Microscopically, follicular dendritic cell tumors are composed of oval to spindle cells with eosinophilic cytoplasm arranged in sheets, fascicles and whorls, sometimes admixed with foci showing a storiform pattern of growth, suggesting a diagnosis of sarcoma. The tumor cells are characteristically admixed with small lymphocytes, leading to an occasional misdiagnosis of lymphoma. The differential diagnosis includes Hodgkin's disease, sarcoma, leiomyosarcoma, gastrointestinal stromal tumor, and inflammatory pseudotumor.9,10 The diagnosis of FDCS requires a high index of suspicion and requires confirmation by immunohistochemical stains. CD21 and CD35 directed against the C3d and C3b receptors are the most commonly used FDCS markers and these stains can be done on formalin-fixed paraffin embedded materials.11 Clusterin and fascin are the recently employed markers.12 The etiology and predisposing factors for FDCS are not ascertained; however, association with Epstein-Barr infection has been suggested, particularly in extranodal FDCS involving the liver.13
Follicular dendritic cell sarcoma is considered to be an indolent tumor with low tendency for recurrence or metastasis. Previous case reports suggest that the clinical course is variable; some patients had a prolonged indolent course, whereas others die as a result of disease progression.6 The behavior of these tumors is more akin to that of a low-grade soft tissue sarcoma than a malignant lymphoma, and is characterized by local recurrence in 36% of cases and metastases in 28%.10
Various treatment modalities have been used to treat this tumor, including surgery, chemotherapy and radiation therapy. Complete surgical resection is the treatment of choice whenever feasible. Adjuvant radiotherapy or chemotherapy appears to be indicated in cases having adverse pathological features and in recurrent or incompletely resected lesions. Cases with microscopic features such as significant cytological atypia, extensive coagulative necrosis, high proliferative index (mitotic count of >5/10 hpf), or tumor size greater than 6 cm have poor prognosis, whereas lesions arising in the lymph nodes behave as low-grade sarcoma with a relatively good prognosis.11 No definite therapeutic approach has demonstrated consistent efficacy.8 In this case, CT scan of the whole abdomen showed two large heterogeneously enhancing masses in the ileocecal region. The patient underwent excision of the masses but did not receive any adjuvant treatment since the tumor was completely resected with adequate margin. No recurrence was detected after one year of follow-up.
Conclusion
Follicular dendritic cell sarcoma should be considered as a differential diagnosis of spindle cell tumor like gastrointestinal stromal tumor, where complete surgical resection seems to be curative without necessitating any adjuvant therapy; however, follow-up is essential in the case of FDC sarcoma.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
Reference
1. Khalid T, Folman R. Symptoms in cancer patients and an unusual tumor: Case 3. Follicular dendritic cell sarcoma. J Clin Oncol 2005 Dec;23(36):9425-9426.
2. Geerts A, Lagae E, Dhaene K, Peeters M, Waeytens A, Demetter P, et al. Metastatic follicular dendritic cell sarcoma of the stomach: a case report and review of the literature. Acta Gastroenterol Belg 2004 Apr-Jun;67(2):223-227.
3. Kang TW, Lee SJ, Song HJ. Follicular dendritic cell sarcoma of the abdomen: the imaging findings. Korean J Radiol 2010 Mar-Apr;11(2):239-243.
4. Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol 1986 Mar;122(3):562-572.
5. Bai LY, Kwang WK, Chiang IP, Chen PM. Follicular dendritic cell tumor of the liver associated with Epstein-Barr virus. Jpn J Clin Oncol 2006 Apr;36(4):249-253.
6. Grogg KL, Lae ME, Kurtin PJ, Macon WR. Clusterin expression distinguishes follicular dendritic cell tumors from other dendritic cell neoplasms: report of a novel follicular dendritic cell marker and clinicopathologic data on 12 additional follicular dendritic cell tumors and 6 additional interdigitating dendritic cell tumors. Am J Surg Pathol 2004 Aug;28(8):988-998.
7. Fonseca R, Yamakawa M, Nakamura S, van Heerde P, Miettinen M, Shek TW, et al. Follicular dendritic cell sarcoma and interdigitating reticulum cell sarcoma: a review. Am J Hematol 1998 Oct;59(2):161-167.
8. Kairouz S, Hashash J, Kabbara W, McHayleh W, Tabbara IA. Dendritic cell neoplasms: an overview. Am J Hematol 2007 Oct;82(10):924-928.
9. Chen TC, Kuo TT, Ng KF. Follicular dendritic cell tumor of the liver: a clinicopathologic and Epstein-Barr virus study of two cases. Mod Pathol 2001 Apr;14(4):354-360.
10. Perez-Ordoñez B, Rosai J. Follicular dendritic cell tumor: review of the entity. Semin Diagn Pathol 1998 May;15(2):144-154.
11. Chan JK, Fletcher CD, Nayler SJ, Cooper K. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer 1997 Jan;79(2):294-313.
12. Grogg KL, Macon WR, Kurtin PJ, Nascimento AG. A survey of clusterin and fascin expression in sarcomas and spindle cell neoplasms: strong clusterin immunostaining is highly specific for follicular dendritic cell tumor. Mod Pathol 2005 Feb;18(2):260-266.
13. Arber DA, Weiss LM, Chang KL. Detection of Epstein-Barr Virus in inflammatory pseudotumor. Semin Diagn Pathol 1998 May;15(2):155-160.
|