|
Abstract
Objectives: This study was aimed to determine plasma levels of total (TFPI-T) and free (TFPI-F) tissue factor pathway inhibitor, plasminogen activator inhibitor-1 (PAI-1), and tissue plasminogen activator (t-PA) in a cohort of Saudi patients with chronic stable angiographically defined coronary artery disease (CAD) and to determine its correlation with its severity.
Methods: This cross sectional study was conducted in the department of physiology and department of cardiology, College of Medicine, and King Khalid University Hospital and King Saud University, Riyadh. Sixty known cases of CAD who had undergone angiography (35 males and 25 females) were selected. A control group included 39 (20 males and 19 females) healthy subjects. Fasting venous blood samples were analyzed for total (TFPI-T) and free (TFPI-F) tissue factor pathway inhibitor, plasminogen activator inhibitor-1 (PAI-1), and tissue plasminogen activator (t-PA). Gensini scores and vessel scores were determined for assessing CAD severity.
Results: There were non-significant differences between age, body mass index (BMI) and Blood pressure between the controls and CAD subjects. A comparison of hemostatic markers between control and CAD patients showed significantly higher levels of Fibrinogen, PAI-1, TFPI-T and TFPI-F in CAD patients compared to control subjects. But there was no difference in plasma t-PA levels. TFPI-T had a significant positive correlation with severity of disease determined by Gensini Scores (r=0.344; p=0.006) and vessel scores (r=0.338; p=0.015).
Conclusion: Plasma levels of total tissue factor pathway inhibitor are significantly related with the presence and severity of CAD. Elevated levels of TFPI-T may be considered as useful diagnostic and prognostic markers in patients with CAD.
Keywords: Coronary artery disease; Hemostasis; Gensini score; Angiography total tissue factor pathway inhibitor; Free tissue factor pathway inhibitor; Plasminogen activator inhibitor-1; Tissue plasminogen activator.
Introduction
A large body of evidence links hemostatic variables to a higher risk of myocardial infarction and stroke.1 Tissue factor (TF) is the major trigger of the coagulation cascade and leads to the activation of the coagulation cascade. Therefore, its inhibition by pharmacological or genetic interventions may be an attractive target for the treatment and prevention of cardiovascular diseases.2 TF mediated activation of the coagulation cascade is inhibited by tissue factor pathway inhibitor (TFPI). Free tissue factor pathway inhibitor (TFPI-F) has been reported to be an important biomarker of myocardial damage.3 In a study by Morange et al plasma TF levels were predictive of cardiovascular mortality in individuals with acute coronary syndromes (ACS), whereas TFPI-F levels were associated with the severity of myocardial damage on admission, but were not independently related to the outcome of these diseases.4 Free TFPI directly inhibits factor Xa,5 whereas surface-bound TFPI on endothelial cells inhibits TF and factor VIIa complex.6,7 Because TFPI is degraded by thrombolytics, a decreased anticoagulant activity may contribute to rethrombosis.8 Activation of the fibrinolytic system by thrombolytic therapy in AMI decreases plasma TFPI. Reduced TFPI may contribute to thrombotic complications after fibrinolysis in AMI.9 Significant relationships have been observed between markers of endothelial damage, thrombomodulin and TFPI.10 TF indeed appears to be involved in the pathogenesis of neointima formation. Therefore, therapeutic strategies are under investigation to specifically interfere with the action of TF or, alternatively, promote the effects of TFPI.2,11-13 Clinical applications of such markers need to be tested in appropriate trials, in particular for evaluating the advantages of targeted versus systemic delivery of the inhibitors. This study was aimed to determine plasma levels of total (TFPI-T) and free (TFPI-F) tissue factor pathway inhibitor, plasminogen activator inhibitor-1 (PAI-1), and tissue plasminogen activator (t-PA) in a cohort of Saudi patients with chronic stable angiographically defined coronary artery disease (CAD) and to determine its correlation with its severity.
Methods
This study was conducted at the department of physiology, College of Medicine and King Khalid University Hospital and King Saud University, Riyadh. The project was conducted from June 2008 to August 2010 and was funded by the College of Medicine Research Center (CMRC).
The study protocol was approved by the ethical Committee of College of Medicine Research Center. The individuals selected were informed about the objectives of the study and those patients who agreed to participate signed the consent form. A clinical record of each individual including personal data, demographic data, family history and results of the coronary angiography were filled in a predesigned proforma.
The inclusion criteria included adult patients of any sex with CAD, who had attacks of Angina or Myocardial infarction and underwent coronary angiography. Patients with nephrotic syndrome, acute or chronic renal failure, thyroid disorders, acute infections, stroke, diabetic ketoacidosis and non-ketotic hyperosmolar diabetes were excluded. In the control group, there were 39 healthy individuals matched for gender, age and BMI. All control subjects were free from clinical manifestations of coronary, peripheral or cerebral artery disease by history, physical examination and electrocardiographic findings.
A total of 60 patients completed the study. Overnight fasting blood samples were collected in sodium citrate tubes and plasma were separated within two to three hours after collection and stored at -80ºC until assayed. The control group had no past history of chest pain, family history or other evidence of CAD.
All patients underwent coronary angiography via the right femoral approach. Evidence of CAD presence was defined on the basis of at least 50% stenosis in a major coronary vessel. Gensini scoring system was used to determine the CAD severity. This scoring system considers the percentage and site of blockage and gives a score to each vessel under consideration. By assessing percentage of luminal narrowing and localization CAD, severity was graded.14 The left main coronary artery, left anterior descending artery, circumflex and right coronary arteries were assessed. Multiple lesions in the same vessel were regarded as one-vessel disease. Vessel scoring was graded into single, double and triple vessel disease.
The plasma concentration of total (TFPI-T) and free (TFPI-F) tissue factor pathway inhibitor-1, plasminogen activator inhibitor 1 (PAI-1), and tissue plasminogen activator (t-PA) were measured by standard sandwich ELISA technique using commercial kits (Diagnostica STAGO, R.C.S. Nanterre, France).
The data were analyzed by computer software program Statistical Package for Social Sciences (SPSS version 10, Chicago). Differences between age, BMI and Blood pressure between the control and CAD subjects were analyzed by Student’s t-test. However, TFPI-T and TFPI-F, PAI-1, and t-PA because of their extreme skewness, were analyzed by non parametric Mann-Whitney U test. A p value of ≤0.05 was considered as statistically significant. Univariate and multiple regression analysis and Spearman’s correlation coefficients were determined between hemostatic markers and CAD severity.
Results
Clinical characteristics of all control and CAD patients are expressed in Table 1. There were non-significant differences between age, BMI and Blood pressure between the controls and CAD subjects. Table 2 expresses comparison of hemostatic markers between the control and CAD patients. There were significantly higher levels of Fibrinogen (0.0253), PAI-1 (0.0466), TFPI-T (0.0001) and TFPI-F (0.0004) in CAD patients compared to control subjects. But the difference in plasma tPA (0.2811) levels was no significant. The mean Gensini score in all CAD patients was 76.99±6.75. CAD patients consisted of 17 subjects with single vessel disease, 20 subjects with double vessel disease, and 23 subjects with triple vessel disease. Table 3 shows Spearman’s correlations between Gensini Scoring, vessel scoring and hemostatic risk markers. It was observed that among different markers of hemostasis, only TFPI-T had a significant positive correlation with severity of disease determined by Gensini Scores (r=0.344; p=0.006) and vessel scores (r=0.338; p=0.015). Furthermore, the relationship with other markers was not significant. However, in multiple regression analysis, predictive power of TFPI-T for CAD severity became non significant (r=0.271; p=0.096).
Table 1: Comparison of demographic and clinical data of control and CAD patients.
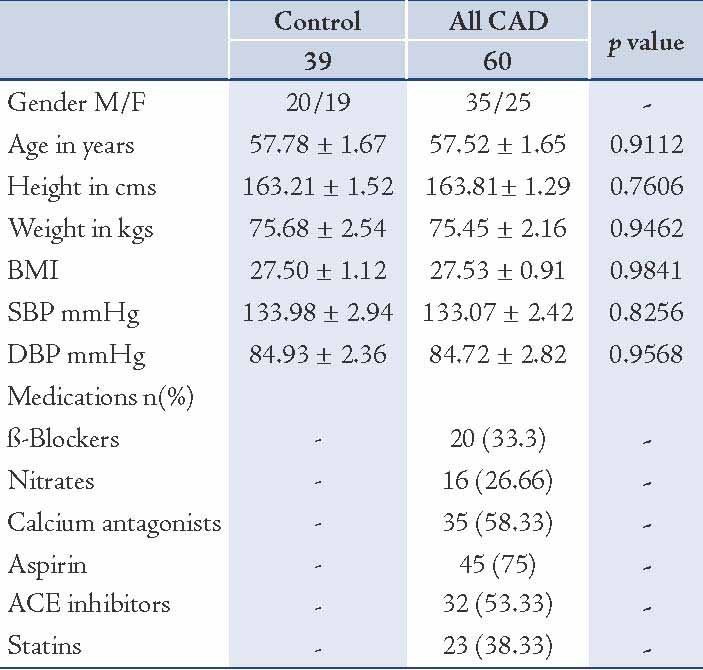
Table 2: Comparison of plasma levels of hemostatic variables between control and CAD patients.
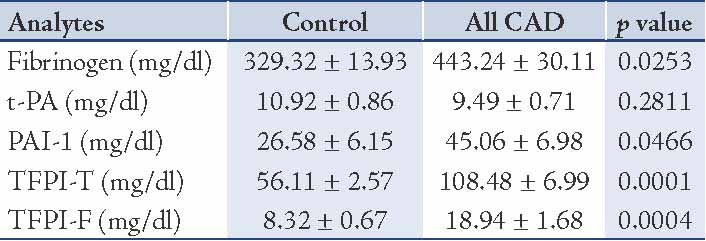
Table 3: Spearman’s correlations between Gensini Scoring, vessel scoring and cardiovascular risk markers.
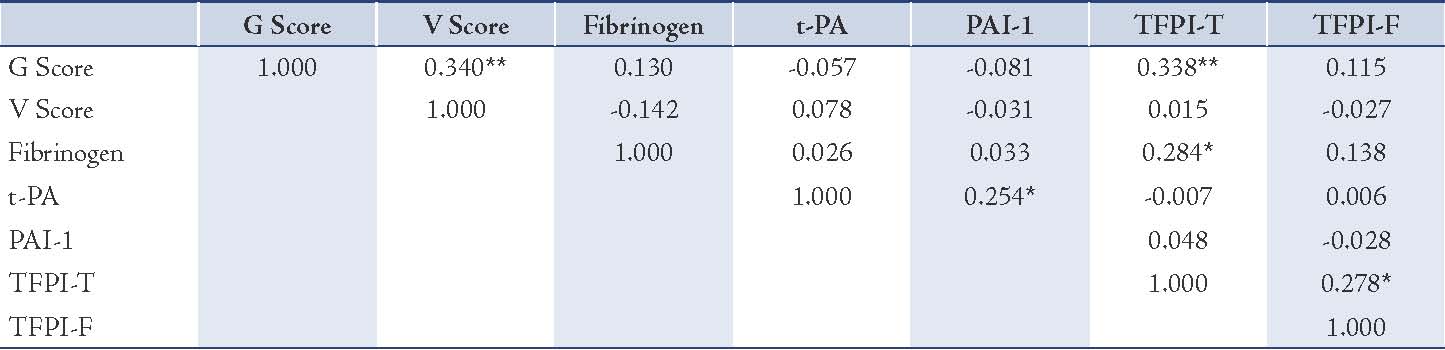
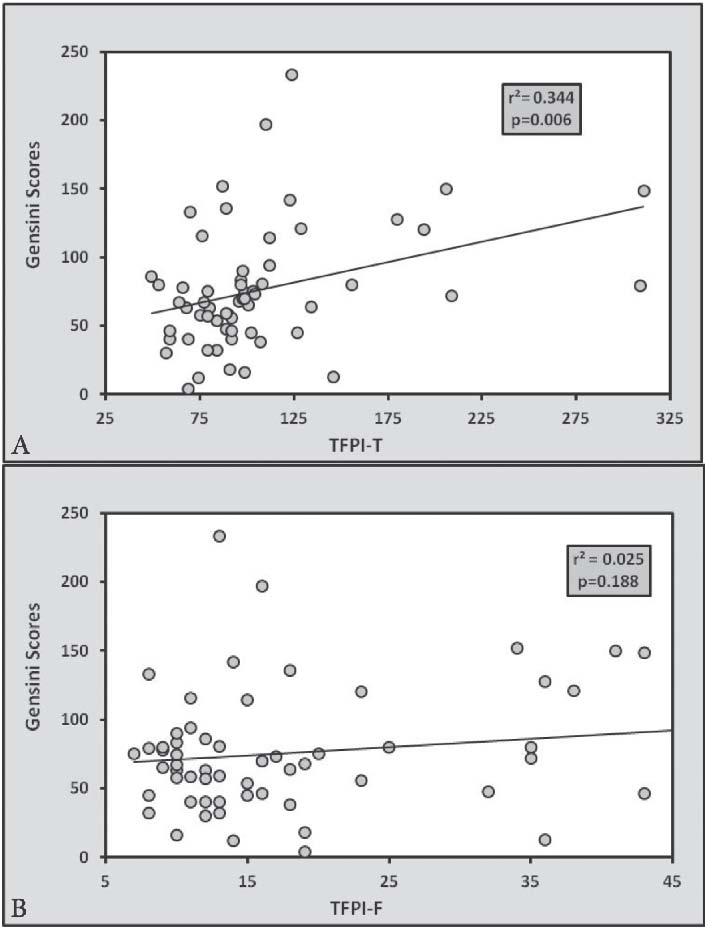
Figure 1: Linear regression analysis showing relationship of Gensini Scoring with (a) TFPI-T and (b) TFPI-F. There is significant relationship of CAD severity with TFPI-T but not with TFPI-F.
Discussion
In this study, we determined the relationship of markers of hemostasis with the presence and severity of angiographically defined CAD. Increased levels of TF have been detected in unstable angina, correlating with the presence of large areas rich in macrophages and smooth muscle cells (SMCs).15 Therefore, the macrophage-rich atherosclerotic plaques (soft plaques), which have a high risk for rupture,16 are at the same time prone to severe thrombosis because of the high expression of TF foam cells.17 Our findings reinforce previous reports by Kaikita et al. suggesting that the presence of TFPI within the luminal surface of atherosclerotic lesions without disruption may play an active role in the prevention of thrombotic complications.11 Morange et al. reported that high TFPI-F levels were not independently associated with an increased risk of cardiovascular death although it correlated significantly with its severity and concluded that only plasma TF levels were predictive of cardiovascular mortality in individuals with ACS.6 The fact that TFPI co-localizes with TF on endothelial cells, smooth muscle cells and macrophages, and inhibits TF procoagulant activity serves as evidence that TFPI inhibits TF activity in the atherosclerotic vessel.18 In a similarly designed study, TF and TFPI plasma levels and FVII coagulant activity were assessed in T2DM in relation to cardiothrombotic disease and their correlation to metabolic and clinical behavior of the patients. The authors reported significantly higher levels of TF and TFPI in diabetics without CAD and also with CAD. Moreover, there were significant correlations between high TF, TFPI plasma levels, FVIIa activity and cardiothrombotic complications in diabetic patients.19
Reports also showed that serum Lp(a) and plasma total TFPI levels are elevated in patients with CAD. Caplice et al.20,21 showed that Lp(a) inhibits TFPI in vitro and concluded that Lp(a) binds TFPI with much higher affinity than plasminogen with which it shares structural similarity. Lp(a) also inactivates TFPI activity in the presence or absence of physiologic plasminogen concentrations. More than 90% of plasma TFPI is associated with lipoproteins, including Lipoprotein(a), LDL, and HDL.7,22 In our studied patients, we found higher levels of total TFPI, but not free TFPI. There are some controversial reports in the literature regarding the relationship of TFPI with CAD severity. Lima et al. found a significant correlation between TF and CAD severity but no correlation was observed with TFPI. However, Bilgen and colleagues reported a significant relationship between TFPI and CAD severity determined by vessel scoring.23,24
The associations of plasma levels of some hemostatic variables like fibrinogen, Von Willebrand factor, t-PA and Lp(a) with CHD risk are reported to be attenuated when inflammatory markers and conventional risk factors are included in multivariable analyses. Moreover, D-dimer and IL-6 each have the potential to increase the prediction of CHD, in addition to conventional risk factors.25
The limitation of the current study is its cross sectional design and small sample size. Long term prospective trials are needed to determine the exact predictive value of these cardiovascular risk markers.
Conclusions
Plasma levels of total tissue factor pathway inhibitor are significantly related with the presence and severity of CAD. Elevated levels of TFPI-T may be considered as useful diagnostic and prognostic markers in patients with CAD.
Acknowledgements
The author is thankful to the College of Medicine Research Center for supporting this project. Special thanks to Antar Al Omani, Mujeeb ul Haq, Aboud and Ester for their cooperation and technical support. The author has no conflicts of interest.
References
1. Saraf S, Christopoulos C, Salha IB, Stott DJ, Gorog DA. Impaired endogenous thrombolysis in acute coronary syndrome patients predicts cardiovascular death and nonfatal myocardial infarction. J Am Coll Cardiol 2010 May;55(19):2107-2115.
2. Holy EW, Tanner FC. Tissue factor in cardiovascular disease pathophysiology and pharmacological intervention. Adv Pharmacol 2010;59:259-292.
3. Toschi V. Soluble tissue factor and tissue factor pathway inhibitor in cardiovascular disease. J Thromb Haemost 2007 Mar;5(3):472-474.
4. Morange PE, Blankenberg S, Alessi MC, Bickel C, Rupprecht HJ, Schnabel R, et al; Atherogene Investigators. Prognostic value of plasma tissue factor and tissue factor pathway inhibitor for cardiovascular death in patients with coronary artery disease: the AtheroGene study. J Thromb Haemost 2007 Mar;5(3):475-482.
5. Broze GJ Jr. Tissue factor pathway inhibitor. Thromb Haemost 1995 Jul;74(1):90-93.
6. Lupu C, Goodwin CA, Westmuckett AD, Emeis JJ, Scully MF, Kakkar VV, et al. Tissue factor pathway inhibitor in endothelial cells colocalizes with glycolipid microdomains/caveolae. Regulatory mechanism(s) of the anticoagulant properties of the endothelium. Arterioscler Thromb Vasc Biol 1997 Nov;17(11):2964-2974.
7. Ott I, Miyagi Y, Miyazaki K, Heeb MJ, Mueller BM, Rao LV, et al. Reversible regulation of tissue factor-induced coagulation by glycosyl phosphatidylinositol-anchored tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol 2000 Mar;20(3):874-882.
8. Hamuro T, Kido H, Asada Y, Hatakeyama K, Okumura Y, Kunori Y, et al. Tissue factor pathway inhibitor is highly susceptible to chymase-mediated proteolysis. FEBS J 2007 Jun;274(12):3065-3077.
9. Ott I, Malcouvier V, Schömig A, Neumann FJ. Proteolysis of tissue factor pathway inhibitor-1 by thrombolysis in acute myocardial infarction. Circulation 2002 Jan;105(3):279-281.
10. Huang J, Fu G, Yao Q, Cheng G. Relation of thrombomodulin, TFPI and plasma antioxidants in healthy individuals and patients with coronary heart disease. Acta Cardiol 2008 Jun;63(3):341-346.
11. Kaikita K, Takeya M, Ogawa H, Suefuji H, Yasue H, Takahashi K. Co-localization of tissue factor and tissue factor pathway inhibitor in coronary atherosclerosis. J Pathol 1999 Jun;188(2):180-188.
12. Breitenstein A, Camici GG, Tanner FC. Tissue factor: beyond coagulation in the cardiovascular system. Clin Sci (Lond). 2009;26;118(3):159-72.
13. Holy EW, Tanner FC. Tissue factor in cardiovascular disease pathophysiology and pharmacological intervention. Adv Pharmacol 2010;59:259-292.
14. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983 Feb;51(3):606.
15. Moreno PR, Bernardi VH, López-Cuéllar J, Murcia AM, Palacios IF, Gold HK, et al. Macrophages, smooth muscle cells, and tissue factor in unstable angina. Implications for cell-mediated thrombogenicity in acute coronary syndromes. Circulation 1996 Dec;94(12):3090-3097.
16. Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, et al. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol 1996 Oct;7(5):330-335.
17. Petit L, Lesnik P, Dachet C, Moreau M, Chapman MJ. Tissue factor pathway inhibitor is expressed by human monocyte-derived macrophages : relationship to tissue factor induction by cholesterol and oxidized LDL. Arterioscler Thromb Vasc Biol 1999 Feb;19(2):309-315.
18. Caplice NM, Mueske CS, Kleppe LS, Simari RD. Presence of tissue factor pathway inhibitor in human atherosclerotic plaques is associated with reduced tissue factor activity. Circulation 1998 Sep;98(11):1051-1057.
19. El-Hagracy RS, Kamal GM, Sabry IM, Saad AA, Abou El Ezz NF, Nasr HA. Tissue Factor, Tissue Factor Pathway Inhibitor and Factor VII Activity in Cardiovascular Complicated Type 2 Diabetes Mellitus. Oman Med J 2010 Jul;25(3):173-178.
20. Caplice NM, Panetta C, Peterson TE, Kleppe LS, Mueske CS, Kostner GM, et al. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: a novel link between lipoproteins and thrombosis. Blood 2001 Nov;98(10):2980-2987.
21. Habib SS, Abdel-Gader AM, Kurdi MI, Al-Aseri Z, Soliman MM. Lipoproteina(a) is a feature of the presence, diffuseness, and severity of coronary artery disease in Saudi population. Saudi Med J 2009 Mar;30(3):346-352.
22. Lwaleed BA, Bass PS. Tissue factor pathway inhibitor: structure, biology and involvement in disease. J Pathol 2006 Feb;208(3):327-339.
23. Lima LM, Sousa MO, Dusse LM, Lasmar MC, das Graças Carvalho M, Lwaleed BA. Tissue factor and tissue factor pathway inhibitor levels in coronary artery disease: correlation with the severity of atheromatosis. Thromb Res 2007;121(2):283-287.
24. Bilgen D, Sönmez H, Ekmekçi H, Ulutin T, Oztürk Z, Kökoğlu E, et al. The relationship of TFPI, Lp(a), and oxidized LDL antibody levels in patients with coronary artery disease. Clin Biochem 2005 Jan;38(1):92-96.
25. Woodward M, Rumley A, Welsh P, MacMahon S, Lowe G. A comparison of the associations between seven hemostatic or inflammatory variables and coronary heart disease. J Thromb Haemost 2007 Sep;5(9):1795-1800.
|