|
Abstract
Dermatofibrosarcoma protuberans (DFSP) is a rare tumor which usually presents during early or middle adult life, as erythematous indurated firm subcutaneous nodules. DFSP involving the breast is a rare phenomenon and even rarer in males. We describe a case of a male patient who presented with this tumor in his left breast.
Keywords: Breast tumor, Dermatofibrosarcoma protuberans, Immunohistochemistry, Male.
Introduction
Dermatofibrosarcoma protuberans (DFSP) is an intermediate grade tumor of fibroblastic origin arising from dermis. This rare tumor usually presents during early or middle adult life as erythematous indurated firm subcutaneous nodules.1 The predominant sites of this tumor are trunk (47.4%) and extremities (38.1%).2 DFSP involving breast is a rare phenomenon and even rarer in males. We describe a case of a male patient who presented with this tumor in his left breast.
Case Report
A 22 year old male patient presented with a gradually increasing lump in his left breast for last 3 years with pain off and on for the last 2 months. It was not associated with any nipple discharge, reduced appetite or weight loss. No significant medical history was present. On clinical examination, a 5 × 5 centimeter non tender, firm lump was palpable, occupying both the superior quadrants, parts of outer inferior quadrant and subareolar region. (Figure 1). The lump was fixed to the skin but free from deeper tissues. Overlying skin was nodular, erythematous with prominent superficial veins. No ulcer was present. Ipsilateral axillary lymph nodes were not palpable and a provisional clinical diagnosis of sarcoma was made.
Fine needle aspiration (FNA) cytology yielded good cellularity smears composed of dense clusters of bland appearing spindle cells of myofibroblastic differentiation with overlapping nuclei, scant cytoplasm and few areas of myxoid degeneration. (Figure 2). A diagnosis of low grade fibromyxoid sarcoma was made and a core needle biopsy was advised.
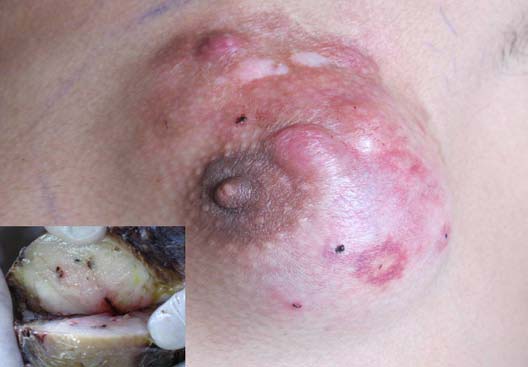
Figure 1: Clinical examination showed a firm palpable lump, occupying both the superior quadrants, parts of outer inferior quadrant and subareolar region. Inset shows cut section view of nodular grayish firm white mass with specks of congestion.
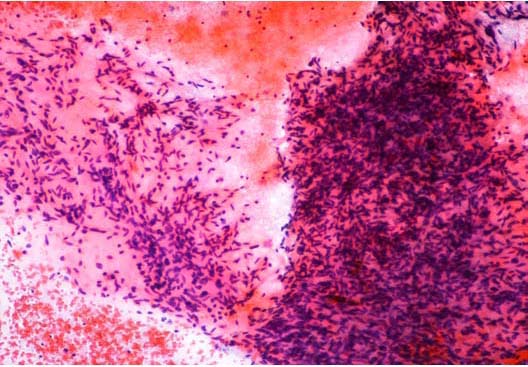
Figure 2: FNA cytology shows dense clusters of bland appearing spindle cells of myofibroblastic differentiation with overlapping nuclei, scant cytoplasm and few areas of myxoid degeneration. Haematoxylin & Eosin × 10.
Core needle biopsy done from the breast lesion showed bland spindle cells resembling fibroblasts that were haphazardly arranged in bundles. No abnormal mitosis or pleomorphism was noted. The diagnosis went in favour of low grade spindle cell tumor of the breast.
Subsequently, the entire tumor was surgically removed along with partial excision of the underlying pectoralis major muscle and ipsilateral axillary lymph nodes. On gross examination, the specimen showed a skin covered soft tissue piece measuring 12 × 10 × 3.5 cm with multiple raised nodules, of which the largest measured 1.2 cm in diameter. Cut section revealed a nodular grayish firm white mass behind nipple-areola of size 6.5 × 6 × 3.8 cm, with specks of congestion.(Figure 1-inset) Four axillary lymph nodes were dissected of which the largest measured 0.8 × 0.6 cm while the smallest measured 0.4 × 0.2 cm.
On microscopy, Haematoxylin and Eosin stained sections from the tumor showed spindle cells in fascicles, long sweeping bundles arranged in focal storiform as well as herringbone pattern with collagen bundles in between and infiltration of tumor cells into subcutaneous fatty tissue (Figure 3). 1-2 mitoses per high power field was observed in the cellular areas. No epithelial or ductal cells were seen. Necrosis and multinucleated giant cells were absent. Axillary lymph nodes and pectoralis major were free of any tumor infiltration. Immunohistochemistry showed positivity for CD34 in tumor cells, (Figure 4) while smooth muscle actin, S-100 and BCL-2 were negative. Thus a final diagnosis of DFSP was made. Our patient is doing well after 18 months of follow up post surgery, without any evidence of tumor recurrence.
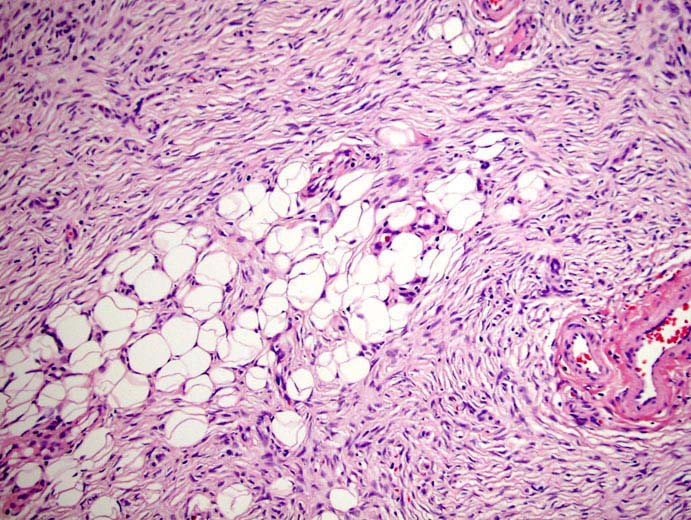
Figure 3: DFSP: Tissue section from the tumor showed spindle cells in fascicles, long sweeping bundles arranged in focal storiform as well as herringbone pattern with collagen bundles in between and infiltration of tumor cells into subcutaneous fatty tissue. Haematoxylin & Eosin × 40.
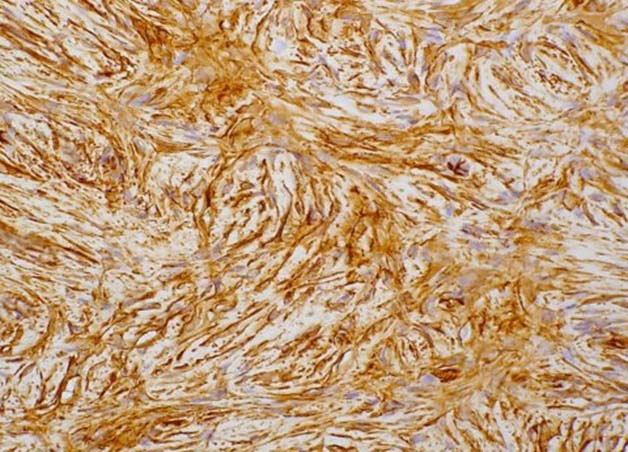
Figure 4: Immunohistochemistry shows CD34 positivity in tumor cells.
Discussion
Dermatofibrosarcoma protuberans (DFSP) is an uncommon intermediate grade malignancy of skin arising from fibroblastic cells in dermis and accounts for 1.8% of all skin tumors.3 This slow growing tumor has a preponderance to occur in young adult males. Clinically it presents as nodular firm subcutaneous mass with a reddish purple hue which may ulcerate subsequently.1 In this currently reported case, the tumor presented as a gradually increasing nodular telangiectatic breast lump in a 22 year old male patient.
Although DFSP most commonly occurs in the trunk and extremities, other areas of the body where this tumor has been reported include the hand,4 gluteal region, head and neck,5 toe,6 vulva7 and the parotid gland. Breast is a very rare site of occurrence of this tumor.
FNAC has been recommended as the first line investigation in evaluating superficial soft tissue tumors.8 Cytology of DFSP usually shows three dimensional aggregates of densely packed short spindle cells with plump ovoid nuclei, scanty cytoplasm and minimal atypia with absence of necrosis and haemorrhage, as was seen in our case. Sometimes branching capillary vessels rimmed by the tumor cells may also be present.8
Histopathologically, the hallmark of DFSP is spindle cells arranged in storiform pattern with minimal atypia, few mitotic figures and absence of necrosis. Immunohistochemically, DFSP shows strong positivity for CD34, vimentin and negative staining for cytokeratin, S-100, epithelial membrane antigen and variable staining for smooth muscle actin (SMA).1 The present case showed similar histopathological features. Positivity for CD34 and negativity for S-100 and SMA helped us to clinch the diagnosis in this case.
DFSP is a locally aggressive tumor with tendency to recur after excision. Hence, wide margin of excision of 3 to 5 cm has been recommended.9 It rarely metastasizes, if at all, then to regional lymph nodes and lungs.1 Though our case presented with tumor infiltration into the subcutaneous fatty tissue with no metastasis to axillary lymph nodes or the lungs, a wide and deep surgical excision performed, has led to disease free survival 18 months post-surgery in our patient.
Conclusion
The main differential diagnosis of DFSP of breast include primary breast tumors with spindle cell differentiation like phyllodes tumor and cellular fibroadenoma, benign fibrous histiocytoma, neurofibroma, nodular fascitis, dermatofibrosarcoma, fibrosarcoma and inflammatory myofibroblastic tumor.1,2,10 Absence of ductal and epithelial structures in the present case pointed towards a mesenchymal origin of the tumor. The typical storiform arrangement of spindle cells with lack of giant cells and strong positivity for CD34 minimized the possibility of benign fibrous histiocytoma. The histology of the tumor and S-100 negativity did not support neurofibroma. As the tumor in our case was of slow growing nature and had a myxoid background with absence of frequent mitotic figures, the diagnosis of nodular fascitis was not favoured. Lack of necrosis, atypia and abundant mitotic figures went against dematofibrosarcoma and fibrosarcoma. Paucity of inflammation and negative staining for smooth muscle actin ruled out the possibility of inflammatory myofibroblastic tumor.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Elder DE, Elenitsas R, Johnson B, Murphy GF. George Xu. Lever's Histopathology of the Skin, 10th ed. Lippincott Williams & Wilkins.2005; p. 355-356.
2. Weiss SW, Goldblum JR. Enzinger & Weiss’s Soft Tissue Tumours. 5th ed. Mosby Elsevier. 2008; p. 371-385.
3. Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer 2004 Dec;101(11):2503-2508.
4. Chiang W, Wang CC, Ho WD, Keh DC, Chung MT. Dermatofibrosarcoma protuberans of the hand: a case report. Zhonghua Yi Xue Za Zhi (Taipei) 1993 Feb;51(2):148-150.
5. Laskin WB. Dermatofibrosarcoma protuberans. CA Cancer J Clin 1992 Mar-Apr;42(2):116-125.
6. Kraemer BA, Fremling M. Dermatofibrosarcoma protuberans of the toe. Ann Plast Surg 1990 Oct;25(4):295-298.
7. Leake JF, Buscema J, Cho KR, Currie JL. Dermatofibrosarcoma protuberans of the vulva. Gynecol Oncol 1991 Jun;41(3):245-249.
8. Koss LG. Koss' Diagnostic Cytology & Its Histopathologic Basis. 5th ed. Lippincott Williams & Wilkins.2006; p 1306-1307.
9. Parker TL, Zitelli JA. Surgical margins for excision of dermatofibrosarcoma protuberans. J Am Acad Dermatol 1995 Feb;32(2 Pt 1):233-236.
10. Tse GM, Tan PH, Lui PC, Putti TC. Spindle cell lesions of the breast–the pathologic differential diagnosis. Breast Cancer Res Treat 2008 May;109(2):199-207.
|