Cervical cancer is one of the most common cancers found in women worldwide, especially in developing countries such as Indonesia. The 2012 GLOBOCAN data show that cervical cancer was the fourth most common cancer in women after breast, colorectal, and lung cancers. These data include an estimated 528 000 new cases and 266 000 deaths, and a five-year-prevalence of 1 547 161 cases.1 The cancer registry of Himpunan Onkologi Ginekologi Indonesia reported 1474 cervical cases in 2013; 67.7% were categorized as locally advanced stage cancer (stage IIB–IVA), and 39.1% were stage IIIB cancer.2
The primary choice of treatment for advanced stage cervical cancer is radiation, but radioresistance has recently become a problem. The rates of treatment failure for post pelvic radiation of cervical cancer are about 10% for stage IB, 17% for stage IIA, 23% for stage IIB, 42% for stage III, and 74% for stage IVA. The rates of recurrence are 58% at one year and 7% at two years.3
Research has identified the characteristics of stem cells found in cancer tissue, which are referred to as cancer stem cells (CSCs). CSCs represent a special subpopulation found in tumor tissue that exhibits the potential for self-renewal and pluripotency. CSCs are thought to be responsible for tumorigenesis, metastasis, resistance to therapy, and tumor recurrence.4–7 Markers of CSCs include Sry-related HMG box (SOX2) and octamer binding transcription factor 4 (OCT4), which are thought to regulate the apoptosis pathway (via caspase-3), telomerase function (via human telomerase reverse transcription or hTERT), and DNA damage repair (via checkpoint kinase 1 or Chk1).8–11
The purpose of this study was to assess these markers and determine whether their expression levels provide clinical information useful for predicting the response to radiotherapy.
Methods
This case-control study focused on the role of CSCs in stage IIIB cervical cancer and the response to treatment (radiation). This study was performed in the oncology clinic of the Obstetrics and Gynecology Department, Radiotherapy Department, and Pathological Anatomy Department of Faculty of Medicine Universitas Indonesia/Cipto Mangunkusumo Hospital from September 2015 to November 2016. Forty-eight patients were included. The inclusion criteria were patients with stage IIIB cervical cancer diagnosed according to the International Federation of Obstetrics and Gynecology criteria and histopathologically proven, completion of radiotherapy or chemoradiotherapy, adequate paraffin blocks, and follow-up by physical examination and Pap smear to monitor cancer recurrence for a minimum of six months and a maximum of 12 months after therapy (total sampling). Patients with a double primary tumor, inaccessible data, or inadequate paraffin blocks for immunohistochemical analysis were excluded. This study was approved by the ethics committee of the Faculty of Medicine, Universitas Indonesia, and informed consent was obtained from all patients.
Each paraffin block was cut into 4 μm thick sections on a microtome, after which, the sections were placed on poly-l-lysine-coated glass slides, which were then heated on a hot plate at 55–58 °C. The samples were deparaffinized in a graded series of xylol and rehydrated in a graded series of alcohol. The slides were subjected to heat-induced antigen retrieval using 0.1 M NaOH citrate buffer (pH 7.0) in an autoclave at 121 °C for 15 minutes and washed with phosphate-buffered saline (PBS; pH 7.4) for five minutes. Endogenous peroxidase was blocked with hydrogen peroxide in methanol (3% v/v) for 30 minutes at room temperature. The slides were then washed under running tap water for five minutes, and nonspecific proteins were blocked with Background Sniper (Biocare Medical) for 15 minutes.
The slides were incubated overnight with primary antibodies to OCT4 (Santa Cruz Biotechnology; dilution 1:150), SOX2 (Santa Cruz Biotechnology; 1:300), or hTERT (Abcam; 1:3000). Some slides were incubated with antibodies to caspase-3 (Abcam; 1:200) and Chk1 (Abcam; 1:1000) for one hour. After the appropriate incubation, the slides were washed with PBS for five minutes, and for SOX2, OCT4, and hTERT staining, the slides were left overnight and then washed with PBS for five minutes.
Each slide was incubated with the appropriate biotinylated secondary antibody (Trekkie Universal Link) for 15 minutes. Diaminobenzene was added, and the slides were incubated for two minutes. The slides were then counterstained with Mayer’s hematoxylin (Lillie’s modified; Roche) for two minutes. Positive and negative controls were included for each antibody.
The immunohistochemistry slides were scanned entirely under low-power magnification (40 ×) on a Leica ICC 50 HD microscope, and 10 representative areas were photographed under high-power magnification (400 ×). The images were evaluated manually using Image J (cell counter) by two pathologists. The expression of SOX2, OCT4, Chk1, caspase-3, and hTERT was evaluated for each patient using an H-score as a semiquantitative approach by determining the staining intensity as strong (3+), moderate (2+), weak (1+) positive, or negative. One thousand cells were examined for each slide to provide a representative score for each sample. The H-score was calculated as (1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+)), which gave a result with a range of 0–300. The mean score for each antibody was used as a cutoff to identify weak or strong expression for each slide.
The patients’ characteristics, radiotherapy response, immunohistochemistry results, and follow-up data were analyzed. The data were analyzed using univariate and inferential statistics and presented as frequencies and percentages for each variable. The data were also analyzed for comparisons using bivariate analysis such as the chi-square test. Multivariate analysis included logistic regression analysis to identify those factors associated with the final therapy response. For all data, a p-value ≤ 0.050 was considered to indicate significance.
Results
This study involved 40 samples from 19 control subjects and 21 patients. Two pathologists performed immunohistochemistry evaluation; their kappa score was 0.750, which indicated good agreement between the two pathologists. The most frequent age group was 45–63 years (72.5%). All samples were stage IIIB cervical cancer with the same type of histopathology (i.e., squamous cell carcinoma (SCC)). All samples were nonkeratinizing SCC because the few keratinized samples were excluded; most (65.0%) were moderately differentiated. All patients received radiation, and four (10.0%) patients received combined radiation and chemotherapy [Table 1].
Table 1: Patients’ characteristics and therapy responses.
|
Age, years |
|
|
|
|
|
< 45 |
2 (13.3) |
3 (12.0) |
3 (14.3) |
2 (10.5) |
|
45–63 |
11 (73.3) |
18 (72.0) |
16 (76.2) |
13 (68.4) |
|
> 63 |
2 (13.3) |
4 (16.0) |
2 (9.5) |
4 (21.1) |
|
Differentiation |
|
|
|
|
|
Well |
1 (6.7) |
5 (20.0) |
3 (14.3) |
3 (15.8) |
|
Moderate |
9 (60.0) |
17 (68.0) |
12 (57.1) |
14 (73.7) |
|
Poor |
5 (33.3) |
3 (12.0) |
6 (28.6) |
2 (10.7) |
|
Therapy |
|
|
|
|
|
Radiation |
12 (80.0) |
24 (96.0) |
18 (85.7) |
18 (94.7) |
aResponse after finishing radiation/chemoradiation. bResponse 6–12 months after therapy.
Table 2: Expression of SOX2 and OCT4, and initial and final therapy responses.
|
SOX2 |
|
|
|
|
|
|
|
|
|
High |
11 (73.3) |
8 (32.0) |
0.011a |
5.80
(1.41–24.17) |
15 (71.4) |
4 (21.1) |
0.001a |
9.40
(2.19–40.11) |
|
Low |
4 (26.7) |
17 (68.0) |
|
|
6 (28.6) |
15 (78.9) |
|
|
|
OCT4 |
|
|
|
|
|
|
|
|
|
High |
13 (86.7) |
8 (32.0) |
0.001a |
13.80
(2.50–76.33) |
17 (81.0) |
4 (21.1) |
< 0.001a |
15.90
(3.38–75.10) |
achi-square test. CSC: cancer stem cell; OR: odds ratio; CI: confidence interval; SOX2: sry-related HMG box; OCT4: octamer binding transcription factor 4.
Table 3: Relationships between SOX2, OCT4, Chk1, caspase-3, and hTERT expression.
|
Chk1 |
|
|
|
|
|
|
|
Low |
17 (81.0) |
13 (68.4) |
0.473b |
15 (78.9) |
15 (71.4) |
0.721b |
|
Strong |
4 (19.0) |
6 (31.6) |
|
4 (21.1) |
6 (28.6) |
|
|
Caspase-3 |
|
|
|
|
|
|
|
Positive |
3 (14.3) |
5 (26.3) |
0.442b |
3 (15.8) |
5 (23.8) |
0.698b |
|
Negative |
18 (85.7) |
14 (73.7) |
|
16 (84.2) |
16 (76.2) |
|
|
hTERT |
|
|
|
|
|
|
|
Low |
11 (52.4) |
9 (47.4) |
0.752a |
8 (42.1) |
12 (57.1) |
0.342a |
achi-square test. bFisher’s exact test. SOX2: sry-related HMG box; OCT4: octamer binding transcription factor 4; Chk1: checkpoint kinase 1;
hTERT: human telomerase reverse transcription.
More than half of the samples (73.3%) from patients with a partial initial treatment response showed strong expression of SOX2 (H-score > 96.6). Similarly, 86.7% of samples from patients with a partial initial treatment response showed strong expression of OCT4 (H-score > 61.9).
A high percentage of samples from patients with an incomplete response to the final treatment exhibited strong expression of SOX2 and OCT4 (71.4% and 81.0%, respectively).
Table 2 shows the expression of SOX2 and OCT4 differed significantly between patients with a partial initial or complete treatment response: SOX2 odds ratio (OR) = 5.80, 95% confidence interval (CI): 1.41–24.17; p = 0.011) and OCT4 (OR = 13.80, 95% CI: 2.50–76.33; p = 0.001). The expression of SOX2 and OCT4 also differed significantly between patients with a partial or complete final treatment response: SOX2 (OR = 9.40, 95% CI: 2.19–40.11; p = 0.001) and OCT4 (OR = 15.90, 95% CI: 3.38–75.10; p < 0.001). Figure 1 shows the immunohistochemical staining of SOX2 and OCT4 expression. Figure 2 shows the patterns of Chk1, caspase-3, and hTERT expression.
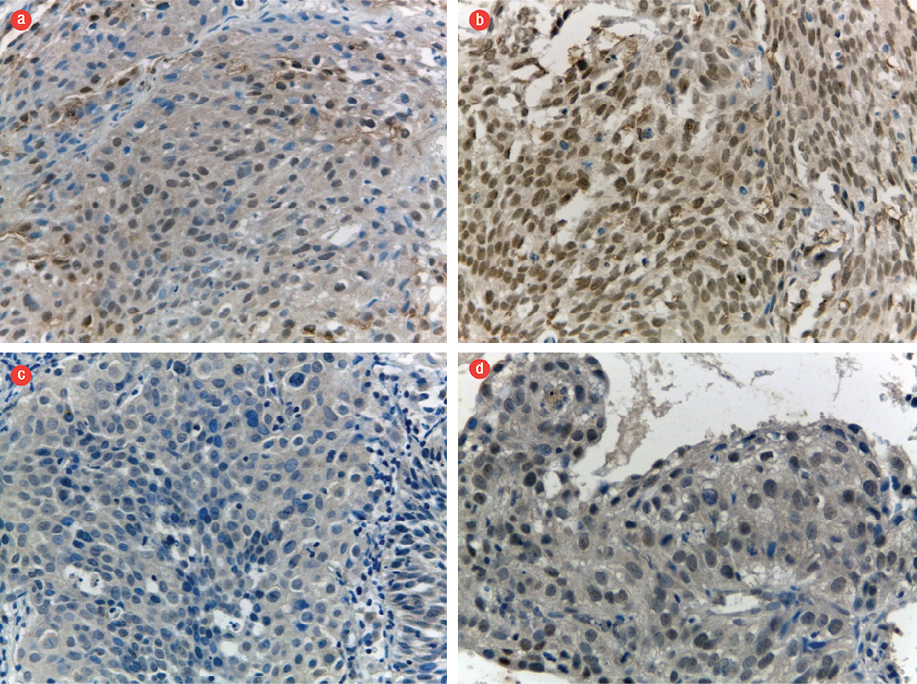
Figure 1: Expression of SOX2 and OCT4. Representative examples with (a) low and (b) high nuclear expression of SOX2, and (c) low and (d) high nuclear expression of OCT4. Magnification = 400 ×.
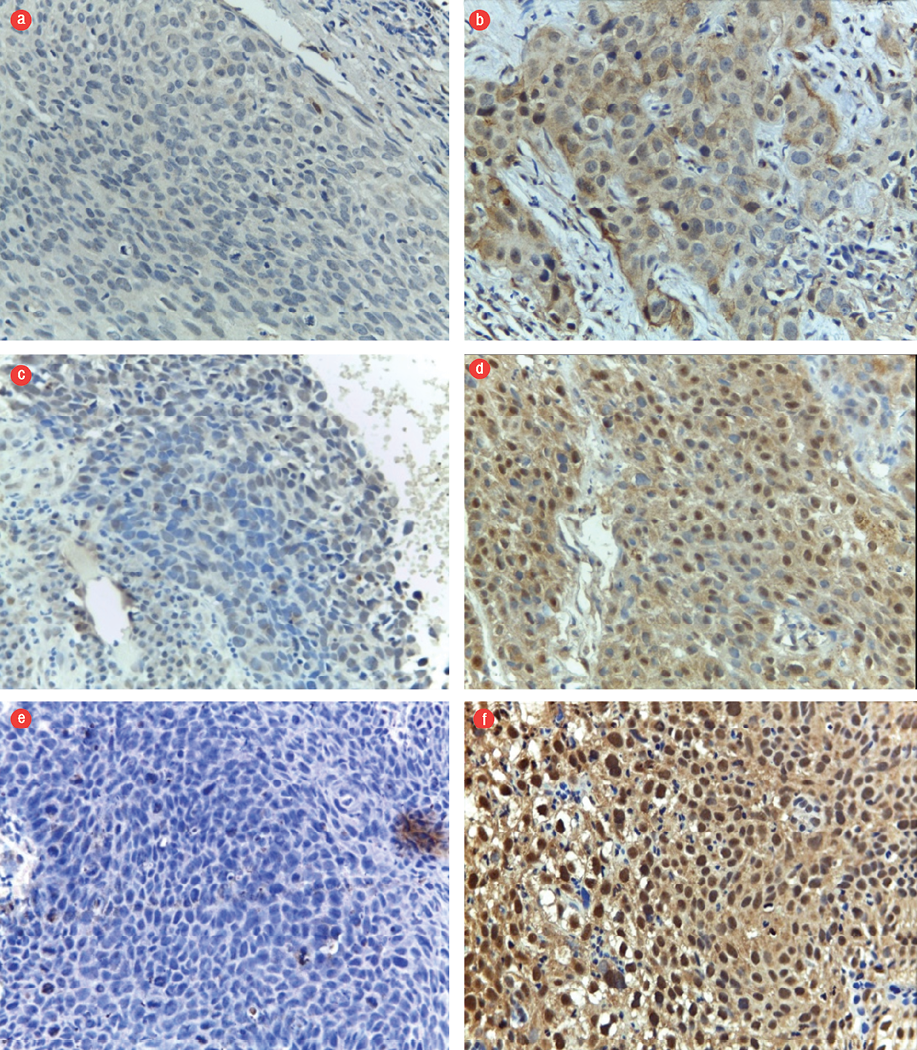
Figure 2: Expression of caspase-3, Chk1, and hTERT. (a) Negative and (b) positive expression of caspase-3 in the cytoplasm. (c) Low and (d) high expression of Chk1 in the nucleus. (e) Low and (f) high expression of hTERT in the nucleus. Magnification = 400 ×.
The expression levels of Chk1, caspase-3, and hTERT as markers of CSCs were compared with those of SOX2 and OCT4 [Table 3]. The expression patterns of these markers did not differ significantly from those of SOX2 and OCT4 (p > 0.050).
Logistic regression multivariate analysis was used to identify the association between markers and the final treatment response. The levels of SOX2 (OR = 5.12, 95% CI: 1.08–24.39; p = 0.034) and OCT4 (OR = 17.03, 95% CI: 3.58–81.15; p = 0.004) expression were significantly associated with the six to 12-month post-radiation response in patients with stage IIIB cervical SCC [Table 4].
The probabilities of exhibiting an incomplete response to the final therapy according to the expression levels of SOX2 and OCT4 were as follows: strong expression of both SOX2 and OCT4, 87.72%; strong expression of OCT4 and weak expression of SOX2, 54.02%; strong expression of SOX2 and weak expression of OCT4, 38.58%; and weak expression of both SOX2 and OCT4, 9.28%.
Six patients (24.0%) had a good initial response to therapy, but this changed to incomplete therapy after 6–12 months because of inguinal and collie lymph nodes metastases, abdominal wall metastases, pleural effusion, and ascites, as confirmed in cytological or histopathological specimens. One patient (4.0%) was diagnosed with SCC based on the Pap smear result.
Table 4: Logistic regression to identify markers of the final therapy response.
|
SOX2 |
9.38 (2.19–40.11) |
0.001 |
5.12 (1.08–24.39) |
0.034 |
|
OCT4 |
15.94 (3.38–75.10) |
< 0.001 |
17.03 (3.58–81.15) |
0.004 |
|
Chk1 |
2.67 (0.58–12.33) |
0.281 |
- |
- |
|
Caspase-3 |
0.60 (0.12–2.94) |
0.698 |
- |
- |
OR: odds ratio; CI: confidence interval; SOX2: Sry-related HMG box; OCT4: octamer binding transcription factor 4; Chk1: checkpoint kinase 1;
hTERT: human telomerase reverse transcription.
Discussion
Cervical cancer is a major health problem in Indonesia. This study included 40 patients with stage IIIB cervical cancer; most patients were aged 45–63 years (72.5%). Histopathologically, all samples were diagnosed as nonkeratinizing SCC. Similar findings were reported in a study from Korea, which reported the most common age group as 19–83 years old and SCC as the most common cancer.12 Previous studies reported that SCC was more frequent in women older than 50 years.13–15 The 45–63 years age range found in our study fits the age range reported by others.13,14
High expression levels of SOX2 and OCT4 are associated with radioresistance of SCC.16 Kumazawa et al,17 also reported morphological differences between the side population (another term for CSC) and non-side population after patients received radiation at a dose of up to 6 Gy. The non-side population colony became separated after radiation treatment. Cells exhibiting positive expression of SOX2 had a greater capacity for self-renewal, differentiation, and tumor formation.18 Some molecular mechanisms may explain the association between CSC existence and radioresistance. The basic principle of radiotherapy has been described as the four “Rs” of radiobiology: repair, redistribution, repopulation, and reoxygenation.19
The CSC response to radio fraction is different from that of non-CSCs. The tumor volume of non-CSC tumors decreases because of DNA damage induced by ionizing radiation. In contrast, CSCs show more active repair of damaged DNA after radiation. The ability of self-renewal through ATM and Chk1/2 is more efficient in CSCs.20 Fast repopulation during and after radiotherapy is an important reason for failed radiotherapy.21,22 CSCs exhibit increased activities of glutamate cysteine ligase and glutathione synthetase. These changes seem to facilitate an increase in the availability of scavengers (i.e., the content of reactive oxygen species and double-strand breaks of DNA is lower in CSCs than in non-CSC populations in response to the same dosage of radiation).20
Apoptosis (via caspase-3), DNA repair (Chk1), and telomerase (hTERT) play roles in carcinogenesis, including that involving SCC. We found no significant differences between expression levels of caspase-3, Chk1, and hTERT, or between those of SOX2 and OCT4, which suggests that the differences in response to therapy might be affected by other molecular factors.23 Clinically, two markers of CSCs—SOX2 and OCT4—should be tested together because each had a different probability of incomplete final therapy response. The probability of DNA repair (Chk1) should also be considered when determining the treatment plan for uterine cervical cancer (e.g., the combination of chemoradiation and an inhibitor of Chk1).24
Conclusion
In patients with keratinizing SCC, expression of SOX2 and OCT4 was significantly associated with a partial initial response to therapy. A high expression level of SOX2 or OCT4 was significantly associated with a partial initial therapy response; the probability of an incomplete final response to therapy was 87.9% in patients with a strong expression of both markers. The patterns of caspase-3, Chk1, and hTERT expression did not differ from those of SOX2 and OCT4. High expression levels of SOX2 and OCT4 suggest an incomplete radiotherapy outcome in patients with stage IIIB cervical cancer.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study. This study was a dissertation research project undertaken in the Faculty of Medicine, Universitas Indonesia. The complete copy of the dissertation was submitted to the Library of the Faculty of Medicine, Universitas Indonesia.
Acknowledgements
We thank all staff members of the Gynecologic Oncology division of the Obstetrics and Gynecology Department, Radiotherapy Department, and Anatomical Pathology Department, Cipto Mangunkusumo Hospital.
references
- GLOBOCAN. Cancer fact sheets: cervical cancer. 2012 [cited 2015 August 01]. Available from: gco.iarc.fr/today/data/pdf/fact-sheets/cancers/cancer-fact-sheets-16.pdf.
- 2. INASGO. Cancer registration information system. 2013 [cited 2015 August 01]. Available from: http://www.inasgo.org/fusionchart/APP/Staging_cervix_bar.asp.
- 3. Andrijono. Kanker serviks. In: Andrijono, Indriatmi W, editors. Infeksi human papilloma virus. Jakarta: Badan Penerbit Fakultas Kedokteran Universitas Indonesia, 2013, p. 119–122.
- 4. Nguyen GH, Murph MM, Chang JY. Cancer stem cell radioresistance and enrichment: where frontline radiation therapy may fail in lung and esophageal cancers. Cancers (Basel) 2011 Mar;3(1):1232-1252.
- 5. Logtenberg ME, Boonstra J. Cancer stem cells and addicted cancer cells. Oncol Discov 2013;1:7.
- 6. Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, et al. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol 2011;2011:941876.
- 7. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997 Jul;3(7):730-737.
- 8. To KK, Fu LW. Multidrug resistance transporters – roles in maintaining cancer stem-like cells. In: Gholamrezanezhad A, editor. Stem cells in clinic and research. InTech; 2011. p. 719-746.
- 9. Weina K, Utikal J. SOX2 and cancer: current research and its implications in the clinic. Clin Transl Med 2014 Jul;3:19.
- 10. Zhang Y, Toh L, Lau P, Wang X. Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/β-catenin pathway in human cancer. J Biol Chem 2012 Sep;287(39):32494-32511.
- 11. Liu H, Liu S, Wang H, Xie X, Chen X, Zhang X, et al. Genomic amplification of the human telomerase gene (hTERC) associated with human papillomavirus is related to the progression of uterine cervical dysplasia to invasive cancer. Diagn Pathol 2012 Oct;7:147.
- 12. Kim BW, Cho H, Choi CH, Ylaya K, Chung J-Y, Kim J-H, et al. Clinical significance of OCT4 and SOX2 protein expression in cervical cancer. BMC Cancer 2015 Dec;15:1015.
- 13. Parkin D, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents Vol. VIII. IARC scientific publication no. 155: Lyon; 2002.
- 14. Parkin DM. The epidemiological basis for evaluating screening policies. In: Franco E, Monsoneg J, editors. New developments in cervical cancer screening and prevention. Oxford: Blackwell Science; 1997. p. 51-69.
- 15. Kumar S, Shah JP, Bryant CS, Imudia AN, Ali-Fehmi R, Malone JM Jr, et al. Prognostic significance of keratinization in squamous cell cancer of uterine cervix: a population based study. Arch Gynecol Obstet 2009 Jul;280(1):25-32.
- 16. Shen L, Huang X, Xie X, Su J, Yuan J, Chen X. High expression of SOX2 and OCT4 indicates radiation resistance and an independent negative prognosis in cervical squamous cell carcinoma. J Histochem Cytochem 2014 Jul;62(7):499-509.
- 17. Kumazawa S, Kajiyama H, Umezu T, Mizuno M, Suzuki S, Yamamoto E, et al. Possible association between stem-like hallmark and radioresistance in human cervical carcinoma cells. J Obstet Gynaecol Res 2014 May;40(5):1389-1398.
- 18. Liu X-F, Yang W-T, Xu R, Liu J-T, Zheng P-S, Bonnet D, et al. Cervical cancer cells with positive Sox2 expression exhibit the properties of cancer stem cells. PLoS One 2014 Jan;9(1):e87092.
- 19. Withers HR. The four R’s of radiotherapy. In: Lett JT, Alder H, editors. Advances in radiation biology. New York: Academic Press; 1975. p. 241–271.
- 20. Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009 Apr;458(7239):780-783.
- 21. Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007 Sep;1(3):313-323.
- 22. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006 Dec;444(7120):756-760.
- 23. Signore M, Ricci-Vitiani L, De Maria R. Targeting apoptosis pathways in cancer stem cells. Cancer Lett 2013 May;332(2):374-382.
- 24. Benada J, Macurek L. Targeting the checkpoint to kill cancer cells. Biomolecules 2015 Aug;5(3):1912-1937.