| |
ABSTRACT
Objectives: Ciprofloxacin is a broad-spectrum antibiotic widely prescribed in clinical and hospital settings. The emergence of antimicrobial resistance against effective antibiotics is a global issue. The objective of study is the surveillance of ciprofloxacin against common pathogens.
Methods: To investigate the present status of antimicrobial resistance against ciprofloxacin, five hundred and twenty four clinical isolates of Escherichia coli (30%), Staphylococcus aureus (33%), Salmonella typhi (9%), Klebsiella pneumonia (14%) and Pseudomonas aeruginosa (14%) were collected during study from January, 2008 to February, 2009 from different pathological laboratories running in and out side hospitals located in Karachi, Pakistan. These pathogens were isolated from specimens of both in and out patients. The in-vitro antimicrobial activity of ciprofloxacin was carried out by Disc Diffusion Method (Kirby-Bauer test).
Results: Showed that ciprofloxacin is 27.02%, 21.95%, 16.66%, 72.22% and 44.44% resistant to Escherichia coli, Staphylococcus aureus, Salmonella typhi, Klebsiella pneumonia and Pseudomonas aeruginosa respectively.
Conclusion: It is concluded that these clinical isolates have started developing resistance against ciprofloxacin due to its irrational and inappropriate use. Continuous surveillance is crucial to monitor the antimicrobial resistance among pathogens.
From the 1Department of Pharmaceutics, Faculty of Pharmacy, University of Karachi; 2Ziauddin College of Pharmacy, Ziauddin University.
Received: 15 Jul 2010
Accepted: 27 Sept 2010
Address correspondence and reprint request to: Dr. Ale Zahra, Ziauddin College of Pharmacy, Ziauddin University, 4/B, Sharah-e-Ghalib, Clifton, Karachi, Pakistan.
E-mail: ale-zehra@hotmail.com
|
|
| |
INTRODUCTION
Antimicrobial resistance (AMR) is a global growing issue and several reports suggest that it is an increasing problem of phenomenal proportions, affecting both developed and developing countries.1-5 AMR is considered as a natural phenomenon for the survival of micro-organism. Therefore, it is imperative to slow the rate of development of AMR to a level that maintains the usefulness of the antimicrobials.6 Accurate determination of bacterial susceptibility to antibiotics is essential for the successful management of bacterial infections and comparative analysis of antimicrobial agents. Public health officials and clinicians monitor drug resistance through appropriate reporting of the results from susceptibility tests and this can be achieved using a number of techniques, including the disk diffusion method, the broth dilution assay, and the E tests.7 As antibiotic resistance reduces treatment efficacy, it is a time to consider routine susceptibility testing to guide individual patient treatment and surveillance of antibiotic resistance.8-10
Ciprofloxacin is one of the fluorinated quinolones structurally related to nalidixic acid. It is a broad spectrum antibiotic, more sensitive to gram- negative bacteria, and less effective against gram-positive bacteria, including Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecalis.11,12 Initially, ciprofloxacin was used to treat infections caused by Gram negative and Gram positives organisms very successfully and resistance was very rare. However, its introduction in the treatment of a broad range of clinical conditions such as the treatment of urinary tract infections, upper respiratory tract infections, and as a prophylaxis for neutropenic patients, as well as its use in veterinary medicine, resistant strains began to emerge.13 Ciprofloxacin is a valuable antibiotic for the empiric treatment of urinary tract infections, nevertheless quinolone’s resistance should be monitored adequately.14 Increasing ciprofloxacin consumption particularly in ICU leads to selection of resistant mutants among nosocomial pathogens since fluoroquinolones are particularly greater selectors of resistance among aminoglycosides, carbapenems, or other ß-lactams and these resistant strains can more easily spread than strains resistant to other drugs.15 Resistance is due to one or more point mutations in the quinolone binding region of the target enzyme or to a change in the permeability of the organism.16
Reduced susceptibility to fluoroquinolones has become a major problem, mostly in Asia.17,18 There are several reports which alert on the customary dispensing of flouroquinolones as over the counter drugs, which may lead to increased resistance of the pathogenic bacteria.19
This study aims to determine the present trends of antimicrobial resistance against ciprofloxacin by different common pathogens. In-vitro disk diffusion method was used to evaluate the growth of inhibition of pathogens, since Bauer-Kirby disk diffusion technique is a simple, reliable, and reproducible way to assess the antimicrobial susceptibilities.20,21
METHODS
524 clinical isolates (Escherichia coli, Staphylococcus aureus, Salmonella typhi, Klebsiella pneumonia and Pseudomonas aeruginosa) were collected from Aga Khan University Hospital, Ehsan-ullah Laboratories and Darusahat Hospital, Karachi, Pakistan. These pathogens were isolated from urine (46%), stool (10%), blood (20%), pus of skin and ear (24%) samples. Ciprofloxacin 6 mm disks (5 µg) were procured from the commercial market (Oxoid Ltd; Basingstoke, Hampshire, England). Antibiotic sensitivity was tested on clinical isolates using Muller-Hinton medium (Oxoid Ltd; Basingstoke, Hampshire, England).
Well- isolated colonies of E. coli, P. aeruginosa, S. aureus, S. typhi and K. pneumonia of same morphological type from an agar plate were selected. Broth was prepared by touching the top of each colony using a sterile wire loop and then transferred it to a tube containing 4 ml to 5 ml of a suitable broth medium, according to the guidelines provided by the National Committee for Clinical Laboratory Standards (NCCLS) for Mueller-Hinton broth and agar medium.
Broth was incubated for 8-24 hours at 37°C. Suspension of bacterial culture having an appropriate turbidity was prepared using 0.5 McFarland standards as a reference so that the number of organisms will be within a given range. A 0.5 McFarland standard was prepared.22 A sterile cotton swab was dipped in the bacterial suspension and then streaked in three directions over the surface of the Mueller-Hinton agar to obtain uniform growth. Mueller-Hinton agar was prepared according to the manufacture’s specifications. Plates were dried for five minutes. Using sterile forceps, place disks of ciprofloxacin 5 µg (CIP 5, Lot No. 634185, expiry date: March, 2011.) on these plates. The discs were placed in such a manner that they were 15 mm from the edge of the plate and not less than 25 mm from each other. Plates were incubated within 15 minutes after applying the disks at 37o C for 24 hours. The diameter of the zones of growth inhibition around each disk was measured to the nearest whole mm using sliding scale. According to the standard values provided by NCCLS these pathogens were classified as sensitive (21 mm), Intermediate resistant (16-20 mm) and resistant (15 mm).23 Intermediate resistant (IR) pathogens were not considered as sensitive or susceptible organisms against the tested antibiotic. The result value ranges are usually regarded as pinpointing of non useful curative option akin to the resistant category for treatment purpose.24
RESULTS
All the clinical isolates were incubated on Muller-Hinton medium with ciprofloxacin (5 µg) disks. These pathogens were isolated from blood, stool, urine, skin, and pus specimens, (Table 1). Organisms lying within the intermediate zones were not considered as sensitive pathogens, because they did not respond to normal therapy. Ciprofloxacin was highly effective against S.typhi and least sensitive against K. pneumoniae. Sensitivity patterns of ciprofloxacin against different pathogens are summarized in Table 2. The value of resistance in percentage of investigated drug is represented in graphical form in Figure 1.
Table 1: Sources of Clinical Isolates
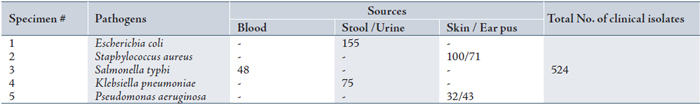
Table 2: Resistance ratio among different clinical isolates against ciprofloxacin
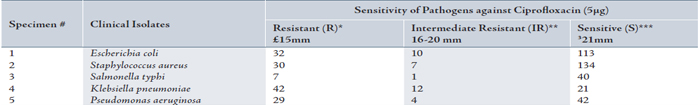
*Resistant (R) means pathogens are not responsive to tested antibiotics.
**Intermediate Resistance (IR) is an indication of non useful therapeutic options similar to the resistant category.
***Susceptible (S) means organisms are responsive to tested antibiotics.24
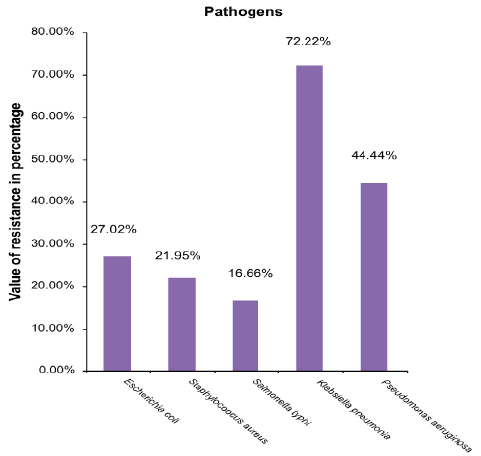
Figure 1: Graphical representation of resistance pattern of clinical isolates against Ciprofloxacin
DISCUSSION
Antibiotic resistance is one of the world’s most pressing public health problems. The antibiotic-resistant organisms can quickly spread and so threaten communities with new strains of infectious disease that are more difficult to cure and more expensive to treat. Treatment failures may arise due to the resistance offered by pathogens against effective broad spectrum antibiotics. These treatment failures and hard-to-treat infections may results in high death rates.25
In this study, routine analysis for development of resistance was done using common pathogens responsible for community infections. E. coli - a Gram negative bacterium and considered as a major source for urinary tract infections, was isolated from urine and stool specimens. It is a good indicator organism of resistance in gram-negative bacteria.26 Ciprofloxacin showed good activity against E. coli, about 27.02% resistance was shown by this pathogen. It was documented in a study conducted at Riyadh hospital that E. coli resistance was increased from 10% in 2001 to 22% in 2005.27 The resistance reported against ciprofloxacin by E. coli ranged between 20-30% in different countries.28,29
Throughout the 1990s, S. aureus acquired more resistance to commonly prescribed antibiotics.30 The clinical isolates of S. aureus in the current study showed less resistance than E .coli. The organisms exhibited 21.95% resistance against ciprofloxacin. This extent of resistance is similar to different studies conducted in different parts of the world. Ciprofloxacin resistance among S. aureus isolates is comparable to that reported in 2000-2002 from United States 51%, Canada 24.1%, Italy 58.6%, Germany 26.1% and France 40.5% respectively.31 There has been an increase in resistance to fluoroquinolones among isolates of S. aureus in recent years. However, the fourth generation fluoroquinolones may reduce this growing and dangerous process of increasing resistance.32
Typhoid fever is a growing concern worldwide.33 World Health Organization (WHO) estimates that there are about 22 million cases of typhoid fever worldwide every year.34 S. typhi is a major cause of typhoid fever. In the present study, the least resistance was shown by S. typhi (16.66%). This percentage of resistance is minimal when compared to the other four pathogens. Ciprofloxacin may be successfully used as a second choice drug after chloramphenicol, the gold standard for typhoid fever.35 Bhattia et al. reported 88% sensitivity of ciprofloxacin in typhoid fever.36 In 1999, at least 10 patients infected with strains with decreased susceptibility to ciprofloxacin did not respond to treatment with fluoroquinolone antimicrobials. However, S. typhi with decreased susceptibility to ciprofloxacin increased to 23% in the U.K. Resistance to ciprofloxacin has been chromosomally encoded in all isolates with decreased sensitivity to this antimicrobial.37
Ciprofloxacin-resistant Salmonella typhi may lead to a situation of untreatable enteric fever.38 In earlier studies, no resistance was reported by S. typhi.39 Since studies to determine the microbial resistances are ongoing with time, it would be interesting to reveal the results of these period studies. The emergence of multi drug-resistant S. typhi has necessitated the use of fluoroquinolones in the therapy of enteric fever.40
Ciprofloxacin is the only available oral antibiotic with anti-pseudomonal coverage. P. aeruginosa is most susceptible to ciprofloxacin and imipenem.41 In the present investigation, it exhibited quite higher resistance than E. coli, S. aureus and S. typhi. It exhibited 44.44% resistance against the tested ciprofloxacin which is in compliance with another study reported by Hu et al. in 2009.42 A latest study affirmed that from 1992 to 2004, P. aeruginosa strains did not show any unusual resistance to the tested agents (piperacillin/tazobactam, ceftazidime, cefepime, ciprofloxacin and tobramycin, meropenem, imipenem, Amikacin,), except for imipenem.43 The resistance to fluoroquinolones is a reflection of mutation, which is a result of selective pressure created by the use of fluoroquinolones.44
In past, fluoroquinolones showed excellent clinical activity against. Enterobacteriaceae including Klebsiella, but the frequency of ciprofloxacin-resistant Klebsiella pneumoniae has increased worldwide in recent years.45,46
In this study, the highest resistance was shown by K. pneumoniae that is 72.22%. This increased resistance showed that K. pneumoniae had adapted to survive in presence of ciprofloxacin and on the basis of these results, it is not considered as an effective therapy choice to eradicate the infections caused by K. pneumonia. The present study is not in agreement with previous data according to which ciprofloxacin was bactericidal and widely used to treat community-acquired pneumonia.47 It is possible that this decreased susceptibility or high-level resistance can be overcome merely by increasing the dosage of ciprofloxacin, analogous to the treatment of intermediate-level penicillin-resistant pneumococci with high-dose penicillin. However, there are no clinical or experimental animal data to support such a high dosage regimen for the treatment of low-level ciprofloxacin-resistant K. pneumoniae or other Enterobacteriaceae.47,48
This study clearly demonstrates the development of resistance for ciprofloxacin by common pathogens especially K. pneumoniae and P. aeruginosa. Initially, ciprofloxacin was highly effective to treat different gram positive and negative bacteria. However, after the passage of time, different factors are attributable for emergence of resistance. These mainly include; high consumption of antibiotics, irrational use, incomplete course of therapy, and self-medication by patients, leading to the emergence of resistance and even treatment failures. One major cause of self-medication is poverty. Pakistan is an under developed country, people are used to treating themselves without obtaining prescriptions from physicians. The present situation is alarming, because it is not long before ciprofloxacin, an effective antibiotic would be failed to treat even simple or minor infections. Curtailed follow up of regimen also creates resistance. Generally patients stop their treatment when they feel slight improvement and thus microorganisms start adapting the environment rather than get killed. Governments must initiates different educational programs, seminars, workshops in collaboration with the media to make people aware of the consequences of self-medication, especially with broad-spectrum antibiotics. In addition to this, routine antimicrobial susceptibility testing must be timely performed to determine the current status of resistance against antimicrobial agents (MIC, E test, Disk diffusion method). Otherwise, therapy failures may occur which increase the cost of the therapy as well as recovery time from the underlying disease.
CONCLUSION
Antimicrobial resistance is a globally ever-increasing problem. Ciprofloxacin is still considered as a better choice against infections caused by E. coli, S. aureus, S. typhi. and K. pneumoniae, which showed highest resistance against ciprofloxacin. The emergence and spread of antimicrobial resistance are complex and driven by numerous interconnected factors. The principle causes of microbial resistance are inappropriate, irrational, high consumption, and profligate use of antibiotics. The use of antimicrobials must be restricted and monitored in order to decline the resistance. Constant surveillance and antibiotic sensitivity testing is vital for patient care and to prevent treatment failures.
ACKNOWLEDGEMENTS
The authors reported no conflict of interest and no funding was received on this work.
|
|
| |
-
Denys GA, Koch KM, Dowzicky MJ. Distribution of resistant gram-positive organisms across the census regions of the United States and in vitro activity of tigecycline, a new glycylcycline antimicrobial. Am J Infect Control October 2007;35(8):521-526.
-
Johnson AP, Pearson A, Duckworth G. Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother September 2005;56(3):455-462.
-
Sharma R, Sharma CL, Kapoor B. Antibacterial resistance: current problems and possible solutions. Indian J Med Sci March 2005;59(3):120-129.
-
Zhang R, Eggleston K, Rotimi V, Zeckhauser RJ. Antibiotic resistance as a global threat: evidence from China, Kuwait and the United States. Global Health 2006;2:6.
-
Felmingham D, Cantón R, Jenkins SG. Regional trends in beta-lactam, macrolide, fluoroquinolone and telithromycin resistance among Streptococcus pneumoniae isolates 2001-2004. J Infect August 2007;55(2):111-118.
-
Sharma R, Sharma CL, Kapoor B. Antibacterial resistance: current problems and possible solutions. Indian J Med Sci March 2005;59(3):120-129.
-
Bonev B, Hooper J, Parisot J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J Antimicrob Chemother June 2008;61(6):1295-1301.
-
Jang CH, Park SY. Emergence of ciprofloxacin-resistant pseudomonas in pediatric otitis media. Int J Pediatr Otorhinolaryngol April 2003;67(4):313-316.
-
Mushtaq MA. What after ciprofloxacin and ceftriaxone in treatment of Salmonella Typhi. Pak J Med Sci 2006;22:51-54.
-
Nweneka CV, Tapha-Sosseh N, Sosa A. Curbing the menace of antimicrobial resistance in developing countries. Harm Reduct J 2009;6:31.
-
Campoli-Richards DM, Monk JP, Price A, Benflied P, Todd PA, Ward A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetics properties and therapeutics use. Drugs 1998;35:373-447.
-
Eliopoulos GM. In vitro activity of fluoroquinolones against gram-positive bacteria. Drugs 1995;49(Suppl 2):48-57.
-
Bazile-Pham-Khac S, Truong QC, Lafont JP, Gutmann L, Zhou XY, Osman M, et al. Resistance to fluoroquinolones in Escherichia coli isolated from poultry. Antimicrob Agents Chemother June 1996;40(6):1504-1507.
-
Lontie ML, Vanerdeweghe PE. Interscience Conference on Antimicrobial Agents and Chemotherapy (43rd: 2003: Chicago).
-
Karlowsky JA, Draghi DC, Jones ME, Thornsberry C, Friedland IR, Sahm DF. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob Agents Chemother May 2003;47(5):1681-1688.
-
Chambers HF. Sulfonamides, Trimethoprim, & Quinolones. In Katzung BG. Basic & Clinical Pharmacology, Boston, 2004. p.777-780.
-
Brown JC, Shanahan PM, Jesudason MV, Thomson CJ, Amyes SG. Mutations responsible for reduced susceptibility to 4-quinolones in clinical isolates of multi-resistant Salmonella typhi in India. J Antimicrob Chemother May 1996;37(5):891-900.
-
Threlfall EJ, Skinner JA, Ward LR. Decreased in vitro susceptibility to ciprofloxacin in resistant Salmonella serotypes typhi and paratyphi A. J Antimicrob Chemother 2001;7(3):448-450.
-
Nagai K, Davies TA, Dewasse BE, Jacobs MR, Appelbaum PC. Single- and multi-step resistance selection study of gemifloxacin compared with trovafloxacin, ciprofloxacin, gatifloxacin and moxifloxacin in Streptococcus pneumoniae. J Antimicrob Chemother September 2001;48(3):365-374.
-
Serrano MC, Ramírez M, Morilla D, Valverde A, Chávez M, Espinel-Ingroff A, et al. A comparative study of the disc diffusion method with the broth microdilution and Etest methods for voriconazole susceptibility testing of Aspergillus spp. J Antimicrob Chemother May 2004;53(5):739-742.
-
Kiehlbauch JA, Hannett GE, Salfinger M, Archinal W, Monserrat C, Carlyn C. Use of the National Committee for Clinical Laboratory Standards guidelines for disk diffusion susceptibility testing in New York state laboratories. J Clin Microbiol September 2000;38(9):3341-3348.
-
Masood H, Naqvi SB, Aslam N. Cost effective analysis of different brands of ceftriaxone available in Karachi, Pakistan. Pak J Pharmacol 2008;25:13-19.
-
CLSI. Performance standards for antimicrobial disk susceptibility tests; Approved standard, 2006, 16th edition. vol 28 (8) M31-A3, Wayne, Pennsylvania, USA., p.1-37.
-
Schwalbe R, Steele-Moore L. Goodwin.AC. Antimicrobial susceptibility testing protocols,2007; Taylor & Francis Group, pp.62
-
Khushal R. Prevalence, characterization and development of resistance pattern in indigenous clinical isolates against cephalosporins.Ph.D Thesis. 2004. Department of Biological Sciences/ Quaid-i-Azam University, Islamabad, Pakistan. p. 1-10.
-
Lontie ML, Van Erdeweghe PE. Sensitivity to Ciprofloxacin of Escherichia coli Isolated from Urine According to Gender and Age. Interscience Conference on Antimicrobial Agents and Chemotherapy (43rd: 2003: Chicago, Ill.).
-
Babay HA. Antimicrobial resistance among clinical isolates of Pseudomonas aeruginosa from patients in a teaching hospital, Riyadh, Saudi Arabia, 2001-2005. Jpn J Infect Dis May 2007;60(2-3):123-125.
-
Glupczynski Y, Delmée M, Goossens H, Struelens M; Belgian Multicenter ICU Study Group. Distribution and prevalence of antimicrobial resistance among gram-negative isolates in intensive care units (ICU) in Belgian hospitals between 1996 and 1999. Acta Clin Belg September-October 2001;56(5):297-306.
-
Pong A, Thomson KS, Moland ES, Chartrand SA, Sanders CC. Activity of moxifloxacin against pathogens with decreased susceptibility to ciprofloxacin. J Antimicrob Chemother November 1999;44(5):621-627.
-
Dajcs JJ, Thibodeaux BA, Marquart ME, Girgis DO, Traidej M, O’Callaghan RJ. Effectiveness of ciprofloxacin, levofloxacin, or moxifloxacin for treatment of experimental Staphylococcus aureus keratitis. Antimicrob Agents Chemother June 2004;48(6):1948-1952.
-
Jones ME, Draghi DC, Thornsberry C, Karlowsky JA, Sahm DF, Wenzel RP. Emerging resistance among bacterial pathogens in the intensive care unit- a European and North American Surveillance study (2000-2002). Ann Clin Microbiol Antimicrob 2004;3:1-11.
-
Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology July 1999;106(7):1313-1318.
-
Mengo DM, Kariuki S, Muigai A, Revathi G. Trends in Salmonella enteric serovar Typhi in Nairobi, Kenya from 2004 to 2006. J Infect Dev Ctries 2010;4(6):393-396.
-
Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ May 2004;82(5):346-353.
-
Krishnan P, Stalin M, Balasubramanian S. Changing trends in antimicrobial resistance of Salmonella enterica serovar typhi and salmonella enterica serovar paratyphi A in Chennai. Indian J Pathol Microbiol October-December 2009;52(4):505-508.
-
Bhatia JK, Mathur AD, Arora MM. Re-emergence of Chloramphenicol Sensitivity in Enteric Fever. MJAFI 2007;63:212-214.
-
Threlfall JE, Ward LR. Decreased Susceptibility to Ciprofloxacin in Salmonella enterica serotype Typhi, United Kingdom. CDC. 2001; 7(3).
-
Gaind R, Paglietti B, Murgia M, Dawar R, Uzzau S, Cappuccinelli P, et al. Molecular characterization of ciprofloxacin-resistant Salmonella enterica serovar Typhi and Paratyphi A causing enteric fever in India. J Antimicrob Chemother December 2006;58(6):1139-1144.
-
Chande C, Shrikhande S, Kapale S, Agrawal S, Fule RP. Change in antimicrobial resistance pattern of Salmonella Typhi in central India. Indian J Med Res June 2002;115:248-250.
-
Rao PS, Rajashekar V, Varghese GK, Shivananda PG. Emergence of multidrug-resistant Salmonella typhi in rural southern India. Am J Trop Med Hyg January 1993;48(1):108-111.
-
M.A. Campos, A. Arias, C. Rodriguez, A. Dorta, L. Betancor, D Loperz-Aguado, et al., Etiology and therapy of chronic suppurative otitis, J Chemother 1995; 7: 427_/431.
-
Hu XH, Xu XM, Mi ZH, Fan YF, Feng WY. [Relationship between drug resistance of Pseudomonas aeruginosa isolated from burn wounds and its mobile genetic elements]. Zhonghua Shao Shang Za Zhi April 2009;25(2):103-105.
-
Fujimura T, Anan N, Sugimori G, Watanabe T, Jinushi Y, Yoshida I, et al. Susceptibility of Pseudomonas aeruginosa clinical isolates in Japan to doripenem and other antipseudomonal agents. Int J Antimicrob Agents December 2009;34(6):523-528.
-
W.H. Sheng, Y.C. Chen, J.T. Wang, S.C. Chang, K.T. Luh, W.C. Hsieh, Emerging fluoroquinolone-resistance for common clinically important gram negative bacteria in Taiwan, Diagn Microbiol Infect Dis 2002; 43: 141_/147.
-
Schumacher H, Scheibel J, Moller JK. Cross-resistance patterns among clinical isolates of Klebsiella pneumoniae with decreased susceptibility to cefuroxime. J Antimicrob Chemother August 2000;46(2):215-221.
-
Thomson CJ. The global epidemiology of resistance to ciprofloxacin and the changing nature of antibiotic resistance: a 10 year perspective. J Antimicrob Chemother March 1999;43(Suppl A):31-40.
-
Fuursted K, Schumacher H. Significance of low-level resistance to ciprofloxacin in Klebsiella pneumoniae and the effect of increased dosage of ciprofloxacin in vivo using the rat granuloma pouch model. J Antimicrob Chemother September 2002;50(3):421-424.
-
Friedland IR, McCracken GH Jr. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med August 1994;331(6):377-382.
|
|