Human immunodeficiency virus (HIV) is increasingly recognized as an important health issue in the Middle East and North Africa (MENA) region.1,2 In 2015, the World Health Organization (WHO) estimated that around 230 000 people were living with HIV in the MENA region with 21 000 new HIV infections and 12 000 acquired immune deficiency syndrome (AIDS)-related deaths.3 This indicates a 35% rise in new HIV cases diagnosed and 66% rise in AIDS-related deaths.4
Routes of HIV transmission vary between countries in the region; some have higher prevalence among intravenous (IV) drug users, while in other countries, transmission is highest among sex workers.5,6 HIV prevention in the MENA region is challenged by many cultural and social barriers, punitive laws, and political unrest, which exacerbate transmission and affect implementation of HIV care.7 In addition, provision of HIV services requires identification of high-risk populations to stop transmission among the general population. This can be extremely difficult without a comprehensive understanding of HIV epidemiology.
In the MENA region, antiretroviral theraphy (ART) coverage remains the lowest; covering only 17% of individuals.7 In addition, access to HIV testing and counseling is still limited.8
Oman is classified as a low prevalence country for HIV/AIDS. This is probably due to religious and cultural practices discouraging premarital and extramarital relations and mandating male circumcision. Nevertheless, the HIV annual detection rate has been reported around 120 to 150 per year indicating a recent increase and reflecting a gap between religious values and believers practice.9 Since 2010, the number of people receiving ART in Oman has increased dramatically and more recently all patients with CD4 lymphocyte count of < 500 cells/mm3 are eligible for ART in concordance with the 2013 WHO guidelines.10 By the end of 2014, 2506 HIV/AIDS cases were reported among Omani nationals, of which 908 were receiving ART.9 In 2015, the Joint United Nations Program on HIV/AIDS (UNAIDS) set the ambitious 90-90-90 target. This global target states that by 2020, 90% of all people living with HIV will know their HIV status, 90% of those diagnosed will receive ART, and 90% of those receiving ART will have viral suppression.11
Despite substantial recent progress in the management of HIV infection in Oman, studies that report epidemiology, clinical characteristics, and outcomes are limited. We sought to review the clinical epidemiology of a cohort of 326 patients presented at a tertiary care hospital in Oman with a focus on our achieved rates on two of the three UNAIDS targets: percentage of HIV patients on treatment, and percentage of patients with viral suppression.
Methods
We conducted an observational review of patients infected with HIV from 1989 to 2016 at the Royal Hospital, Muscat, Oman. Over the 27-year period, 326 patients were identified, all of whom were Omani nationals. Participants included newly diagnosed HIV patients hospitalized at Royal Hospital or diagnosed during antenatal screening, and those referred from other hospitals with treatment failure. The information was obtained from hospital medical records and included demographic data such as geographic origin, age, gender, marital status, education level, mode of transmission, WHO staging, opportunistic infections, laboratory analyses, lymphocyte subset analysis, HIV RNA polymerase chain reaction (PCR), HIV mutations, and ART.
Serologic testing for HIV 1 and 2 was performed using a chemiluminescent microparticle enzyme immunoassay (MEIA, AxSYM HIV 1/2 gO, Abbott Laboratories, Abbott Park, USA). If serology was reactive, specimens were confirmed using the INNO-LIA HIV I/II score (INNOGENETICS NV, Technologiepark, Belgium); a line immunoassay for the confirmation and discrimination of antibodies to HIV-1, HIV group O, and HIV-2 in human serum and plasma. We used the WHO classification and staging of AIDS.12 Lymphocyte subset analysis was obtained by standard flow cytometric methods.
Molecular testing included HIV PCR, which uses three sets of four primers from envelope, polymerase, reverse transcriptase, and core protein genes. Viral load was measured using the branched DNA method (VER SANT HIV-1 RNA 3.0 Assay, [bDNA], Bayer Diagnostics, Berkeley, CA, USA). Results were reported in a range from 20 to > 500 000 IU/mL. HIV genotype resistance testing was performed by PCR amplification and DNA sequencing (HIV-1 GenoSure PRIme, LabCrop, France).
Descriptive statistics were used to summarize the data. For categorical variables, frequencies and percentages were reported. Differences between groups were analyzed using Pearson’s chi-square test. For continuous variables, mean and standard deviation were used to summarize the data. An a priori two-tailed level of significance was set at p < 0.050. Statistical analyses were conducted using SPSS Statistics (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp).
Results
The overall mean age of the cohort was 36.0±15.0 years, and 60.4% (n = 197) were males. The route of transmission in females was predominately by heterosexual contact in 89 (68.9%) patients, prenatal or vertical transmission in 29 (22.5%), and blood transfusion in three (2.3%) patients. There was no data for eight patients (6.2%). In males, transmission was by several routes; 103 patients (52.3%) acquired HIV by heterosexual contact, 24 (12.2%) by prenatal transmission, 20 (10.2%) by homosexual contact, four (2.0%) by IV drug use, and one (0.5%) by blood transfusion. For 45 patients (22.8%), there was no documented data available for transmission route [Table 1].
Table 1: Demographic and clinical characteristics of the cohort.
|
Age, mean ± SD, years |
36.0 ± 15.0 |
33.0 ± 15.0 |
38.0 ± 15.0 |
< 0.001 |
|
Mode of transmission |
|
|
|
|
|
MSM |
20 (6.1) |
0 (0.0) |
20 (10.2) |
< 0.001 |
|
Blood transfusion |
4 (1.2) |
3 (2.3) |
1 (0.5) |
|
Heterosexual |
192 (58.9) |
89 (69.0) |
103 (52.3) |
|
IV drug user |
4 (1.2) |
0 (0.0) |
4 (2.0) |
|
Prenatal |
53 (16.3) |
29 (22.5) |
24 (12.2) |
|
Unknown |
53 (16.3) |
8 (6.2) |
45 (22.8) |
|
Reasons for HIV testing |
|
|
|
|
|
Unknown |
15 (4.6) |
4 (3.1) |
11 (5.6) |
< 0.001 |
|
Antenatal screen |
28 (8.6) |
27 (21) |
1 (0.5) |
|
Postnatal screen |
43 (13.2) |
23 (17.8) |
20 (10.2) |
|
Post transfusion screen |
1 (0.3) |
0 (0.0) |
1 (0.5) |
|
HIV contact |
60 (18.4) |
29 (22.5) |
31 (15.7) |
|
AIDS-related symptoms |
169 (51.8) |
44 (34.1) |
125 (63.5) |
|
Needle stick injury |
1 (0.3) |
0 (0.0) |
1 (0.5) |
|
Pre-employee screening |
5 (1.5) |
2 (1.6) |
3 (1.5) |
|
Prisoner |
3 (0.9) |
0 (0.0) |
3 (1.5) |
|
TB |
1 (0.3) |
0 (0.0) |
1 (0.5) |
|
WHO stage 4 |
|
|
|
|
|
No data |
10 (3.1) |
3 (2.3) |
7 (3.6) |
< 0.001 |
|
No |
231 (70.9) |
107 (82.9) |
124 (62.9) |
|
Yes |
85 (26.1) |
19 (14.7) |
66 (33.5) |
|
HBsAg |
|
|
|
|
|
Negative |
295 (90.5) |
117 (90.7) |
178 (90.4) |
0.658 |
|
Positive |
9 (2.8) |
2 (1.6) |
7 (3.6) |
|
Not done |
22 (6.7) |
10 (7.8) |
12 (6.1) |
|
HCV IgG |
|
|
|
|
|
Negative |
276 (84.9) |
112 (86.8) |
164 (83.7) |
|
Positive |
19 (5.8) |
4 (3.1) |
15 (7.7) |
SD: standard deviation; MSM: men who have sex with men; IV: intravenous; HIV: human immunodeficiency virus; AIDS: acquired immune deficiency syndrome; TB: tuberculosis; WHO: World Health Organization; HBsAg: surface antigen for hepatitis B; HCV IgG: test for hepatitis C. Percentages might not add up to 100% due to rounding off.
The WHO has established a four-stage classification system based on opportunistic infections and other HIV related outcomes. In our cohort, 169 patients (51.8%) presented with HIV related symptoms and 85 patients (26.1%) had WHO stage 4 HIV symptoms (i.e., AIDS). Our data also showed that the rates of HIV/HBV and HIV/HCV coinfections were 2.8% and 5.8%, respectively [Table 1].
At the time of first hospital visit, male patients presented with lower CD4 + cells (< 200 cells/mm3) than females (46.2% vs. 25.6%; p = 0.003). However, there were no significant differences in HIV RNA titers (> 1000 copies/mL3) between genders (76.1% vs. 72.1%; p = 0.136) [Table 2].
Low-level HIV viremia ranging from 21 to 1000 IU/mL was seen in 48 (14.7%) patients. Twenty-four (7.4%) patients had undetected HIV viral load on initial presentation (0–20 IU/mL). Those patients were transferred from other institutes for either treatment continuation with their partners or further antenatal care.
At the time of analysis, only 50 (15.3%) patients had CD4+ < 200 cells/mm3 while 257 (78.8%) had CD4+ values > 200 cells/mm3, and no data was available for 19 (5.8%) patients indicating a good immunological response to ART. Complete HIV RNA viral suppression was seen in 169 (51.8%) patients. Sixty-four (19.6%) HIV infected patients had low-level viremia ranging from 21 to 1000 IU/mL, while complete virological failure was seen in 81 (24.8%) patients. HIV genotype resistance testing was performed in 165 (50.6%) patients. HIV resistance testing was mostly done in treatment failures and treatment naïve patients particularly pregnant females.
Table 2: Clinical outcome characteristics of the cohort.
|
Genotype resistance test |
|
|
|
|
|
No |
161 (49.4) |
56 (43.4) |
105 (53.3) |
0.081 |
|
Yes |
165 (50.6) |
73 (56.6) |
92 (46.7) |
|
Baseline CD4 count, cells/mm3 |
|
|
|
|
|
< 200 |
124 (38.0) |
33 (25.6) |
91 (46.2) |
0.003 |
|
201–500 |
99 (30) |
46 (35.7) |
53 (26.9) |
|
> 500 |
88 (27.0) |
43 (33.3) |
45 (22.8) |
|
No data* |
15 (4.6) |
7 (5.4) |
8 (4.1) |
|
Latest CD4 count, cells/mm3 |
|
|
|
|
|
< 200 |
50 (15.3) |
12 (9.3) |
38 (19.3) |
0.009 |
|
201–500 |
112 (34.4) |
39 (30.2) |
37.1) |
|
> 500 |
145 (44.5) |
73 (56.6) |
72 (36.5) |
|
No data* |
19 (5.8) |
5 (3.9) |
14 (7.1) |
|
Baseline HIV viral load, IU/mL |
|
|
|
|
|
0–20 |
24 (7.4) |
15 (11.6) |
9 (4.6) |
0.136 |
|
21–1000 |
48 (14.7) |
18 (14.0) |
30 (15.2) |
|
> 1000 |
243 (74.5) |
93 (72.1) |
150 (76.1) |
|
No data* |
11 (3.4) |
3 (2.3) |
8 (4.1) |
|
Latest HIV viral load, IU/mL |
|
|
|
|
|
0–20 |
169 (51.8) |
71 (55.0) |
98 (49.7) |
0.734 |
|
21–1000 |
64 (19.6) |
24 (18.6) |
40 (20.3) |
|
> 1000 |
81 (24.8) |
31 (24.0) |
50 (25.4) |
|
No data* |
12 (3.7) |
3 (2.3) |
9 (4.6) |
|
On ART |
|
|
|
|
|
No |
12 (3.7) |
5 (3.9) |
7 (3.6) |
0.977 |
|
Yes |
314 (96.3) |
124 (96.1) |
190 (96.4) |
|
Outcome |
|
|
|
|
|
Alive |
275 (84.4) |
116 (89.9) |
159 (80.7) |
|
Dead |
22 (6.7) |
4 (3.1) |
18 (9.1) |
HIV: human immunodeficiency virus; ART: antiretroviral therapy. Percentages might not add up to 100% due to rounding off.
ART was given to 314 (96.3%) patients. Twelve patients (3.7%) either refused or deferred therapy. Among patients on ART, 35.0% (n = 110) of the patients received ART prior to 2009 while 65.0% (n = 204) received ART after the implementation of universal HIV treatment policy in 2010.
The most common ART regimen was non-nucleoside reverse transcriptase inhibitor-based therapy with nucleoside reverse transcriptase inhibitors backbone that included either combination of lamivudine/zidovudine or tenofovir disporoxil/emtricitabine. Tenofovir disoproxil based regimens were widely used after 2010. The most common protease inhibitors were lopinavir/ritonavir and atazanavir/ritonavir. Integrase inhibitors (INIs) have been used most recently as a second-line alternative for treatment failures.
Among a total of 326 HIV infected patients, 6.7% (n = 22) died and 8.9% (n = 29) were lost to follow-up. The annual mortality rate was 0.342 per 1000 per year for the last five years [Figure 1].
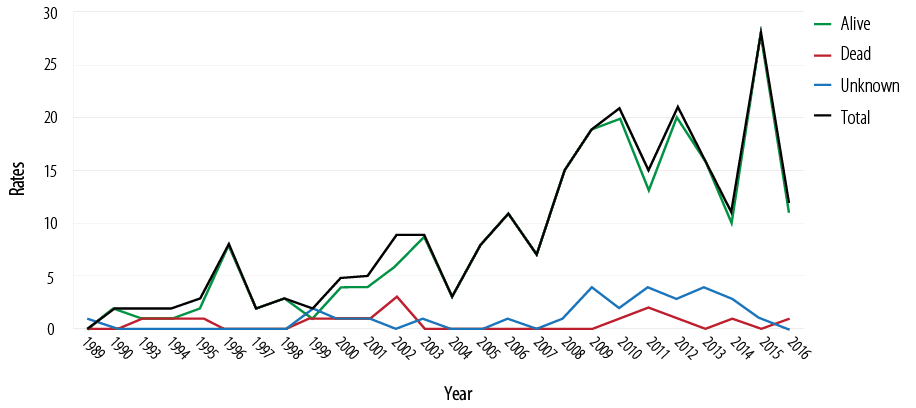
Figure 1: Survival and mortality rates of 326 HIV-1 patients over the last 27 years.
Discussion
To our knowledge, this is the first study describing the epidemiology, clinical, and laboratory characteristics of a cohort of 326 HIV infected patients and their outcomes in Oman. The most common mode of transmission in both genders was heterosexual contact causing 58.9% of infections. Men who have sex with men (MSM) are among the groups affected by HIV in the region.8,9 However, due to discrimination and social stigma, the true prevalence of HIV in MSM is unknown and probably underestimated with national data suggesting a prevalence of 13.9%, followed by mother-to-child (5.5%), IV drug use (4%), and blood transfusion (2.8%).9 A recent report from the MENA had indicated an increase in HIV acquisition through IV drug users (IVDUs) in countries like Oman, Bahrain, Morocco, Egypt, and KSA.5,13,14 This might be partly explained by the increase in HIV screening programs among IVDUs.
In our cohort, 45 (22.8%) male patients had an unknown mode of transmission, most likely the under-reporting is due to fear of discloser and stigma in particular among MSM population. In addition, a higher rate of mother-to-child transmission has been observed (around 16%) probably representing earlier transmissions when no antenatal screening was done, and management of HIV in pregnancy was suboptimal. Since 2009, a universal screening program for all pregnant females has been taking place in Oman with an acceptance rate of 99%.9
HIV coinfection affects the natural history and clinical outcomes of individuals infected with HBV or HCV virus, resulting in rapid progression to liver cirrhosis and hepatocellular carcinoma.15–17 The prevalence of HBV and HCV coinfection with HIV is not well documented in the region. A systematic review and meta-analysis report on the prevalence of HIV, HBV, HCV, and HIV coinfections in different subpopulations conducted in Iran estimated the prevalence rates of HIV/HBV and HIV/HCV around 1.9% and 10.9%, respectively.18 Similarly, a retrospective analysis of 142 HIV positive patients in Western Saudi Arabia estimated the prevalence of HIV/HBV and HIV/HCV coinfections at 2.8% and 8.5%, respectively.19
The prevalence of HIV/HBV and HIV/HCV coinfection seen was low at 2.8% and 5.8%, respectively. The low prevalence of HIV/HBV coinfection is probably due to the low to intermediate prevalence of HBV in the general population20 and HBV vaccination since 1990. However, a more accurate estimate is required in other high-risk groups, such as IVDUs and prisoners.21
Several studies from the MENA region have reported a correlation of HIV, HBV, and HCV with age and gender.22–25 HBsAg was more common among males in all age groups while anti-HCV was more prevalent among older females.25–28 In contrast, in our study HIV/HCV coinfection was more prevalent in young patients and predominately in men with IVDU risk. HIV/HBV coinfection did not correlate with age or gender.
In Oman, despite free access to healthcare and the significant programmatic improvement in management of HIV infection, patients still present at advanced stages due to the delay in screening and detection of HIV. This was seen in our cohort, where 51.8% of HIV infected patients already presented with symptomatic infection and 26.1% were WHO stage 4 (AIDS) at the time of first hospital visit. It is well established that patients with CD4+ cell counts < 200 cells/mm3 are at higher risk for opportunistic infections, cancers, and death.29–33 The delay in diagnosis can be attributed to several factors such as a lack of HIV awareness among individuals and health care workers, a lack of surveillance of key populations (i.e., MSM or IVDU), a limited number of voluntary counseling and testing clinics, and no access to self HIV testing. In this study, male patients presented with significantly lower CD4 T lymphocytes than female patients (46.2% vs. 25.6%, respectively). This has been observed in several studies where females infected with HIV tend to be younger, have higher CD4 T lymphocyte counts and lower HIV viral loads at the time of initial HIV diagnosis.34–37 However, correlation with transmission rates, clinical outcomes, rate of disease progression, and death has shown contradictory results. In a recent systematic review that included studies from both developed and developing countries, females starting ART had slightly better survival compared to males but showed no clear benefit in progression to either AIDS or to differences in HIV suppression and immunologic recovery.38
The difference in mortality was attributed to several explanations including a protective effect of female sex hormones and higher expression of IFN-stimulated genes in HIV-1-infected women.39 In our setting, early detection in females could be partly attributed to the antenatal screening program that was adopted in the country from 2009.
HIV RNA viral suppression defined as viral load < 20 IU/mL was seen in 169 (51.8%) patients, while 64 (19.6%) patients had viral suppression per WHO criteria (i.e., viral load of < 1000 IU/mL). Complete virological failure was seen in 81 (24.8%) patients. The high virological failure rates can be attributed to de novo drug resistance or lack of adherence and compliance with HIV medications (which is more common in our setting). Nevertheless, as a result of implementing several interventions in our clinic (i.e., counseling on compliance and adherence by trained health care workers, detailed review by clinical pharmacists each visit, using clinical pathways, and HIV resistance testing), the number of patients achieving viral suppression has increased in the last few years, making the UNAIDS target of 90% of HIV patients on treatment virally suppressed feasible by 2020.
The current wide availability of ART and recent changes in international guidelines encouraging early initiation of ART regardless of CD4+ T lymphocytes has lowered morbidity and mortality among HIV infected individuals. The total number of deaths in Oman has decreased from 200 in 2003 to 100 in 2014 (UNAIDS published data 2018). However, the annual mortality rate in our cohort was 0.342 per 1000 person-years in the last five years; significantly higher than mortality rates reported from the developed countries (0.025 deaths per 1000 person-years).40
Among the greater ongoing challenges faced in Oman, are the rises in new HIV cases and AIDS-related mortality rates despite a strong national HIV program and the free access to ART. The increase HIV cases can be attributed to increased screening, better diagnostic modalities, and early detection; however, late presentation with advanced HIV infection and high mortality rates indicate a substantial gap in the cascade of HIV care. Thus, an urgent priority is to reach the UNAIDS target where 90% of all people living with HIV will know their HIV status. The national AIDS program should establish interventions that focus on early detection and preventive strategies. By outreaching key at-risk populations, the program can offer access to health services and engage infected people in a system that enables them to access HIV testing without fear.
One of the main limitations of our study is its cross-sectional design where some data are missing as some patients were lost to follow-up either due to death or transfer to other centers. In addition, despite a large number of patients in our cohort, the study data were collected from a single center, and this might not fully be representative of the country as a whole. Our results may only apply to Oman or countries with similar HIV prevalence rates and cultural background.
Conclusion
The majority of patients acquired HIV through heterosexual transmission. One-quarter of patients in our cohort were diagnosed late with symptoms of HIV related illness and AIDS, 96.3% of the patients were initiated on ART, and 71.5% achieved virological suppression. Among the priorities in Oman are increasing HIV testing and early diagnoses in key at-risk populations, this will help in fast-tracking and linking infected individuals to appropriate services in order to reach the WHO 90-90-90 target by 2020.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
The authors would like to thank the physicians at the Infectious Diseases Unit at the Royal Hospital and the technicians in the Virology and Hematology sections at the Royal Hospital, Muscat, Oman.
references
- UNAIDS. Prevention gap report. 2016 [cited 2017 November 26]. Available from: www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf.
- 2. Abu-Raddad LJ, Hilmi N, Mumtaz G, Benkirane M, Akala FA, Riedner G, et al. Epidemiology of HIV infection in the Middle East and North Africa. AIDS 2010 Jul;24(Suppl 2):S5-S23.
- 3. World Bank Group. Characterizing the HIV/AIDS epidemic in the Middle East and North Africa: Time for strategic action. 2010 [cited 2017 November 26]. Available from: https://openknowledge.worldbank.org/handle/10986/2457.
- 4. Bozicevic I, Riedner G, Calleja JM. HIV surveillance in MENA: recent developments and results. Sex Transm Infect 2013 Nov;89(Suppl 3):iii11-iii16.
- 5. Mumtaz GR, Weiss HA, Thomas SL, Riome S, Setayesh H, Riedner G, et al. HIV among people who inject drugs in the Middle East and North Africa: systematic review and data synthesis. PLoS Med 2014 Jun;11(6):e1001663.
- 6. Mahfoud Z, Afifi R, Ramia S, El Khoury D, Kassak K, El Barbir F, et al. HIV/AIDS among female sex workers, injecting drug users and men who have sex with men in Lebanon: results of the first biobehavioral surveys. AIDS 2010 Jul;24(Suppl 2):S45-S54.
- 7. Mumtaz GR, Riedner G, Abu-Raddad LJ. The emerging face of the HIV epidemic in the Middle East and North Africa. Curr Opin HIV AIDS 2014 Mar;9(2):183-191.
- 8. Gökengin D, Doroudi F, Tohme J, Collins B, Madani N. HIV/AIDS: trends in the Middle East and North Africa region. Int J Infect Dis 2016 Mar;44:66-73.
- 9. HIV Management in Oman: A guide for health care workers. National AIDS Program, Department of Communicable Diseases, Directorate General for Disease Surveillance and Control, Ministry of Health, Oman. 3rd ed. 2015.
- 10. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2016 [cited 2017 December 7]. Available from: www.who.int/hiv/pub/arv/arv-2016/en.
- 11. UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. 2017 [cited 2017 December 7]. Available from: www.unaids.org/en/resources/909090.
- 12. World Health Organization. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance. 2005 [cited 2017 December 7]. Available from: www.who.int/hiv/pub/guidelines/casedefinitions/en.
- 13. Mumtaz G, Hilmi N, McFarland W, Kaplan RL, Akala FA, Semini I, et al. Are HIV epidemics among men who have sex with men emerging in the Middle East and North Africa?: a systematic review and data synthesis. PLoS Med 2010 Aug;8(8):e1000444.
- 14. Al-Mozaini MA, Mansour MK, Al-Hokail AA, Mohmed MA, Daham MA, Al-Abdely HM, et al. HIV-care outcome in Saudi Arabia: a longitudinal cohort. J AIDS Clin Res 2014 Nov;5(11):370.
- 15. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012 May;142(6):1264-1273.e1.
- 16. Michielsen PP, Francque SM, van Dongen JL. Viral hepatitis and hepatocellular carcinoma. World J Surg Oncol 2005 May;3:27.
- 17. Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006;44(1)(Suppl):S6-S9.
- 18. Bagheri Amiri F, Mostafavi E, Mirzazadeh A. HIV, HBV and HCV coinfection prevalence in Iran - a systematic review and meta-analysis. PLoS One 2016 Mar;11(3):e0151946.
- 19. Al-Mughales JA. Co-infection assessment in HBV, HCV, and HIV patients in Western Saudi Arabia. J Med Virol 2016 Sep;88(9):1545-1551.
- 20. Kaminski G, Alnaqdy A, Al-Belushi I, Nograles J, Al-Dhahry SH. Evidence of occult hepatitis B virus infection among Omani blood donors: a preliminary study. Med Princ Pract 2006;15(5):368-372.
- 21. Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016 Jul;16(7):797-808.
- 22. El-Sherif A, Abou-Shady M, Abou-Zeid H, Elwassief A, Elbahrawy A, Ueda Y, et al. Antibody to hepatitis B core antigen as a screening test for occult hepatitis B virus infection in Egyptian chronic hepatitis C patients. J Gastroenterol 2009;44(4):359-364.
- 23. Rebbani K, Ouladlahsen A, Bensghir A, Akil A, Lamdini H, Issouf H, et al. Co-infections with hepatitis B and C viruses in human immunodeficiency virus-infected patients in Morocco. Clin Microbiol Infect 2013 Oct;19(10):E454-E457.
- 24. Heijnen M, Mumtaz GR, Abu-Raddad LJ. Status of HIV and hepatitis C virus infections among prisoners in the Middle East and North Africa: review and synthesis. J Int AIDS Soc 2016 May;19(1):20873.
- 25. Chaabna K, Mohamoud YA, Chemaitelly H, Mumtaz GR, Abu-Raddad LJ. Protocol for a systematic review and meta-analysis of hepatitis C virus (HCV) prevalence and incidence in the Horn of Africa sub-region of the Middle East and North Africa. Syst Rev 2014 Dec;3:146.
- 26. Daw MA, Shabash A, El-Bouzedi A, Dau AA; Association with the Libyan Study Group of Hepatitis & HIV. Seroprevalence of HBV, HCV & HIV co-infection and risk factors analysis in Tripoli-Libya. PLoS One 2014 Jun;9(6):e98793.
- 27. Akhtar A, Khan AH, Sulaiman SA, Soo CT, Khan K. HBV and HIV co-infection: Prevalence and clinical outcomes in tertiary care hospital Malaysia. J Med Virol 2016 Mar;88(3):455-460.
- 28. Chen X, He JM, Ding LS, Zhang GQ, Zou XB, Zheng J. Prevalence of hepatitis B virus and hepatitis C virus in patients with human immunodeficiency virus infection in Central China. Arch Virol 2013 Sep;158(9):1889-1894.
- 29. Miller V, Mocroft A, Reiss P, Katlama C, Papadopoulos AI, Katzenstein T, et al. Relations among CD4 lymphocyte count nadir, antiretroviral therapy, and HIV-1 disease progression: results from the EuroSIDA study. Ann Intern Med 1999 Apr;130(7):570-577.
- 30. Farizo KM, Buehler JW, Chamberland ME, Whyte BM, Froelicher ES, Hopkins SG, et al. Spectrum of disease in persons with human immunodeficiency virus infection in the United States. JAMA 1992 Apr;267(13):1798-1805.
- 31. Hanna DB, Gupta LS, Jones LE, Thompson DM, Kellerman SE, Sackoff JE. AIDS-defining opportunistic illnesses in the HAART era in New York City. AIDS Care 2007 Feb;19(2):264-272.
- 32. Frisch M, Biggar RJ, Engels EA, Goedert JJ; AIDS-Cancer Match Registry Study Group. Association of cancer with AIDS-related immunosuppression in adults. JAMA 2001 Apr;285(13):1736-1745.
- 33. Cheung MC, Pantanowitz L, Dezube BJ. AIDS-related malignancies: emerging challenges in the era of highly active antiretroviral therapy. Oncologist 2005 Jun-Jul;10(6):412-426.
- 34. Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001 Mar;344(10):720-725.
- 35. Nicastri E, Angeletti C, Palmisano L, Sarmati L, Chiesi A, Geraci A, et al; Italian Antiretroviral Treatment Group. Gender differences in clinical progression of HIV-1-infected individuals during long-term highly active antiretroviral therapy. AIDS 2005 Mar;19(6):577-583.
- 36. Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, et al; International Epidemiologic Databases to Evaluate AIDS Southern Africa Collaboration. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med 2012;9(9):e1001304.
- 37. Mosha F, Muchunguzi V, Matee M, Sangeda RZ, Vercauteren J, Nsubuga P, et al. Gender differences in HIV disease progression and treatment outcomes among HIV patients one year after starting antiretroviral treatment (ART) in Dar es Salaam, Tanzania. BMC Public Health 2013 Jan;13:38.
- 38. Castilho JL, Melekhin VV, Sterling TR. Sex differences in HIV outcomes in the highly active antiretroviral therapy era: a systematic review. AIDS Res Hum Retroviruses 2014 May;30(5):446-456.
- 39. Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis 2014 Jul;209(Suppl 3):S86-S92.
- 40. Centers for Disease Control and Prevention. National Center for Health Statistics USA. 2016 [cited 2017 December 7]. Available from: https://www.cdc.gov/nchs/index.htm.