|
INTRODUCTION
Menopause is the permanent cessation of menstruation caused by a decline in ovarian follicular activity; it generally occurs between 35 and 58 years of age.1 Menopause occurs when all the follicles have atrophied. Plasma concentrations of estrogens fall and those of follicle stimulating hormone (FSH) and, to a lesser extent, lutenizing hormone (LH) increases after removal of the negative feedback to the pituitary.2
g-glutamyltransferase (GGT) is an enzyme involved in the transfer of the g-glutamyl residue from g-glutamyl peptides to amino acids, H2O, and other small peptides.3 In most biological systems, glutathione serves as the g-glutamyl donor.3 On the other hand, GGT is also involved in the synthesis of glutathione.4 The intracellular glutathione (GSH) level depends upon the equilibrium between processes during which it is consumed and its biosynthesis is limited by cysteine availability. The metabolism of GSH is closely connected to Meister’s γ-glutamyl cycle, in which a pivotal role is played by membrane GGT enzyme.5 This enzyme participates in the Salvage pathway of extracellular GSH by catalyzing its hydrolysis to amino acid components of cysteine, which is used for intracellular GSH biosynthesis.6 Normally, due to cysteine toxicity, the physiological level of this amino acid in cells is very low, and in plasma cysteine occurs mainly in the form of a disulfide-cystine. Consequently, the importance of the g-glutamyl cycle lies in recovering and delivering cysteine, (Fig. 1). The availability of cysteine necessary for the biosynthesis of cellular glutathione, the most important cell antioxidant, depends upon GGT activity; hence this enzyme may play an important role in the anti-oxidative defense system of the cell.6
The aim of this study is to evaluate the exact role of GGT as a marker of oxidative stress associated with menopause in Iraqi women.
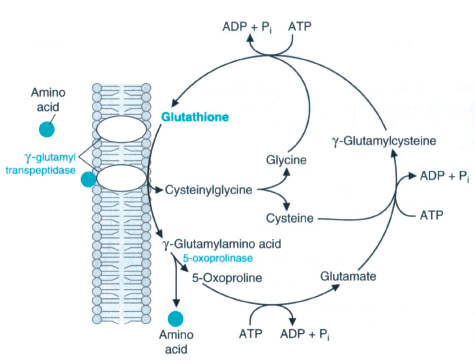
Figure 1: γ-Glutamyl cycle.7
METHODS
The study was carried out at the Chemistry and Biochemistry department, College of Medicine, Al-Nahrain University, Baghdad, Iraq between October 2009 and January 2010. The study was conducted on 33 healthy females, 17 were of pre-menopausal age, (mean=33.64±8.22 years) and 16 were of post-menopausal age (mean=57.58±4.75 years). All subjects were confirmed free of disease on the basis of clinical history, physical examination and routine laboratory tests, and all participants were not on any medical treatment including supplementation of antioxidants or alcohols, and were all free from any hepatobiliary disease.
The protocol for the study was approved by the Ethical committee of Al-Nahrain Medical College, and informed signed consent was given by each subject. Venous blood from different participants was obtained using vein puncture. The blood was centrifuged at 1800 x g for 10 minutes at 4°C and the serum was collected.
The activity of serum GGT was measured according to Szasz in which the substrate L-γ-glutamyl -3- carboxy-4- nitroanilide in the presence of glycylglycine is converted by GGT in the sample to 5-amino-2-nitrobenzoate which can be measured at 405 nm.8 Serum glutathione was measured using the method of Thomas and Skrinska, which is based on the reaction of glutathione with 5,5’- dithiobisnitrobenzoic acid to form a complex that absorbs light at 412 nm. 9 The results were expressed as mmol/L.
Plasma lipid peroxidation was determined using the procedure described by Yoshoiko, in which MDA, an end product of fatty acid peroxidation reacts with thiobarbituric acid (TBA) to form a colored complex with a maximum absorbance at 532 nm.10
Data is presented as mean ±SD. The student’s t-test was performed to assess the statistical significant differences between the two groups.
RESULTS
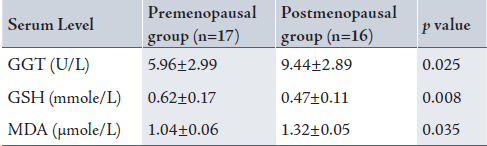
Table 1 shows a significantly higher serum GGT and MDA in the post-menopausal group with a reduction in serum glutathione compared with the pre-menopausal group.
Table 1: Serum γ-glutamyltransferase, glutathione and malondialdehyde levels in the pre- and postmenopausal women.
DISCUSSION
Menopause is known to be associated with a wide variety of physical and psychological symptoms.11
Free radical reactions are involved in processes connected with aging. Estradiol acts as an antioxidant and free radical scavenger, but the mechanism of this action remains unknown.12
The deficiency of estrogen in post-menopausal women is believed to be a factor in the development of oxidative stress, and the release of free radicals or reactive oxygen species (ROS) which becomes the cause of various pathologies.11 This may explain in part, the significantly higher serum MDA levels in the post menopausal group in the present study.
The formation of excessive amounts of reactive oxygen species (ROS), including peroxide (H2O2) and superoxide anions (O2-) is toxic to cells, hence, metabolizing and scavenging systems to remove them are functionally critical and tightly controlled in the cells. Glutathione peroxidase (GPx) in concert with catalase and superoxide dismutase (SOD) function to protect the cell from damage due to ROS.13 Glutathione peroxidase detoxifies peroxides with glutathione acting as electron donor in the reduction reaction, producing oxidized glutathione (GSSG) as an end product.13
The highly significant reduction in glutathione levels observed in the post-menopausal-group in this study could be due to the increase in its free radical scavenging property and increased consumption to counteract the elevated levels of oxidative stress and to inhibit membrane lipid peroxidation.
The significant increase in serum GGT in the post-menopausal group relative to the pre-manopausal group is to compensate and correct the reduced glutathione levels by increasing its biosynthesis.
CONCLUSION
The association of this increase in serum GGT with enhanced oxidative stress and reduced antioxidant defense system in the post-menopausal women may lead to the speculation that GGT could be considered an index or a marker of oxidative stress.
ACKNOWLEDGEMENTS
The authors reported no conflict of interest and no funding was received on this work.
|